A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Hesperetin and Hesperidin Glucoside
2.3. Analysis of Hesperetin, Hesperidin and Hesperidin Glucoside
2.3.1. Determination of Hesperetin, Hesperidin and Hesperidin Glucoside
2.3.2. Identification by FT-IR
2.3.3. Identification by LC-MS
2.4. Determination of Partition Coefficient
2.5. Determination of Antioxidant Activity
2.5.1. DPPH Radical Scavenging Assay
2.5.2. ABTS Radical Scavenging Assay
2.6. Determination of Effects on Inflammatory Mediators and Pro-Inflammatory Cytokines
2.6.1. Cell Culture, Cytotoxicity
2.6.2. Measurement of Inflammatory Mediators
2.6.3. Measurement of Pro-Inflammatory Cytokine Levels
2.7. Determination of Antibacterial Activity
2.7.1. Microorganisms
2.7.2. Determination of Minimum Inhibitory Concentration (MIC)
2.7.3. Determination of Minimal Bactericidal Concentration (MBC)
2.8. Statistical Analysis
3. Results
3.1. Preparation of Hesperetin and Hesperidin Glucoside
3.1.1. Enzymatic Conversion of Hesperidin to Hesperetin
3.1.2. Enzymatic Conversion of Hesperidin to Hesperidin Glucoside
3.2. Identification of Hesperetin and Hesperidin Glucoside
3.2.1. FT-IT Spectra of Hesperetin, Hesperidin and Hesperidin Glucoside
3.2.2. Mass Spectral Fragmentation of Hesperetin, Hesperidin and Hesperidin Glucoside
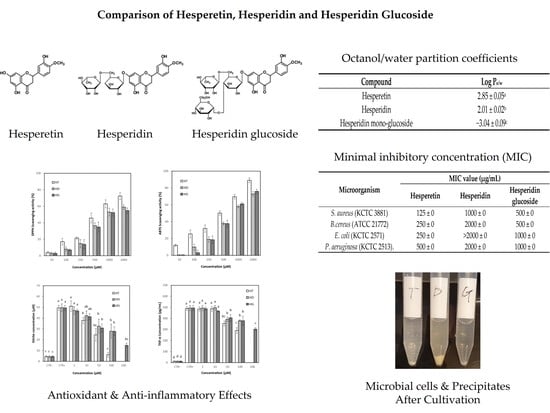
3.3. Partition Coefficient of Hesperetin, Hesperidin and Hesperidin Glucosides
3.4. Antioxidant Activity of Hesperetin, Hesperidin and Hesperidin Glucoside
3.5. Effects of Hesperetin, Hesperidin and Hesperidin Glucoside on Cell Viability
3.6. Effects of Hesperetin, Hesperidin and Hesperidin Glucoside on Inflammation
3.6.1. Effects on NO and PGE2 Levels
3.6.2. Effects on TNF-α and IL-6 Levels
3.7. Antibacterial Activity of Hesperetin, Hesperidin and Hesperidin Glucoside
3.7.1. Effects on MIC
3.7.2. Effects on MBC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Németh, K.; Plumb, G.W.; Berrin, J.-G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell β-glycosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [PubMed]
- Matsumoto, H.; Ikoma, Y.; Sugiura, M.; Yano, M.; Hasegawa, Y. Identification and quantification of the conjugated metabolites derived from orally administered hesperidin in rat plasma. J. Agric. Food Chem. 2004, 52, 6653–6659. [Google Scholar] [PubMed]
- Pereira-Caro, G.; Polyviou, T.; Ludwig, I.A.; Nastase, A.M.; Moreno-Rojas, J.M.; Garcia, A.L.; Malkova, D.; Crozier, A. Bioavailability of orange juice (poly)phenols: The impact of short-term cessation of training by male endurance athletes. Am. J. Clin. Nutr. 2017, 106, 791–800. [Google Scholar] [PubMed] [Green Version]
- Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; González-Sarrías, A.; Espín, J.C. New Insights into the Metabolism of the Flavanones Eriocitrin and Hesperidin: A Comparative Human Pharmacokinetic Study. Antioxidants 2021, 10, 435. [Google Scholar]
- Cho, J. Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch. Pharmacal. Res. 2006, 29, 699–706. [Google Scholar]
- Haidari, F.; Keshavarz, S.A.; Rashidi, M.R.; Shahi, M.M. Orange juice and hesperetin supplementation to hyperuricemic rats alter oxidative stress markers and xanthine oxidoredutase activity. J. Clin. Biochem.Nutr. 2009, 45, 285–291. [Google Scholar]
- Hirata, A.; Murakami, Y.; Shoji, M.; Kadoma, Y.; Fujisawa, S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005, 25, 3367–3374. [Google Scholar]
- Hao, Y.; Wei, Z.; Wang, Z.; Li, G.; Yao, Y.; Dun, B. Biotransformation of flavonoids improves antimicrobial and anti-breast cancer activities in vitro. Foods 2021, 10, 2367. [Google Scholar]
- Olas, B. A review of in vitro studies of the anti-platelet potential of citrus fruit flavonoids. Food Chem. Toxicol. 2021, 150, 112090. [Google Scholar]
- Paredes, A.; Aluzuru, M.; Mendez, J.; Rodriguez-Ortega, M. Anti-sindbis activity of flavanones hesperetin and naringenin. Biol. Pharm. Bull. 2003, 26, 108–109. [Google Scholar]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef] [PubMed]
- Man, M.Q.; Yang, B.; Elias, P.M. Benefits of hesperidin for cutaneous functions. Evid. Based Complement. Altern. Med. 2019, 266307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, R.; Zhao, Y.; Zhou, Z.; Zhao, X. Enhancement of the water solubility and antioxidant activity of hesperidin by chitooligosaccharide. J. Sci. Food Agric. 2018, 98, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Zarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-B-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet flavonoids”: Glycosidase-catalyzed modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, M.; Suzuki, A.; Hase, T. Short-term effects of glucosyl hesperidin and hesperetin on blood pressure and vascular endothelial function in spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2008, 54, 95–98. [Google Scholar] [CrossRef] [Green Version]
- De Souza, V.T.; de Franco, É.P.; de Araújo, M.E.; Messias, M.C.F.; Priviero, F.B.M.; Alexabder, C.H.; Frankland Sawaya, A.C.; de Oliveira Carvalho, P. Characterization of the antioxidant activity of aglycone and glycosylated derivatives of hesperetin: An in vitro and in vivo study. J. Mol. Recognit. 2016, 29, 80–87. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Ahah, S.A.; Khatib, A.; Makhtat, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial effects of flavonoids and their structure-activity relationship: A comparative interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Bellocco, A.; Barreca, D.; Laganà, G.; Leuzzi, U.; Tellone, E.; Ficarra, S.; Kotyk, A.; Galtieri, A. Influence of L-rhamnosyl-D-glucosyl derivatives on properties and biological interaction of flavonoids. Mol. Cell Biochem. 2009, 321, 165–171. [Google Scholar] [CrossRef]
- Cho, E.J.; Li, L.; Yamabe, N.; Kim, H.Y. Antioxidative effects of hesperidin and hesperetin under cellular system. CNU J. Agri. Sci. 2011, 38, 717–722. [Google Scholar]
- Sugasawa, N.; Katagi, A.; Kurobe, H.; Nakayama, T.; Nishio, C.; Takumi, H.; Higashiguchi, F.; Aihara, K.; Shimabukuro, M.; Sata, M.; et al. Inhibition of Atherosclerotic Plaque Development by Oral Administration of α-Glucosyl Hesperidin and Water-Dispersible Hesperetin in Apolipoprotein E Knockout Mice. J. Am. Coll. Nutr. 2019, 38, 15–22. [Google Scholar] [PubMed]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Rémésy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [PubMed] [Green Version]
- Baik, I.-H.; Kim, K.-H.; Lee, K.-A. Antioxidant, Anti-Inflammatory and Antithrombotic Effects of Ginsenoside Compound K Enriched Extract Derived from Ginseng Sprouts. Molecules 2021, 26, 4102. [Google Scholar] [PubMed]
- Choi, S.-S.; Park, H.-R.; Lee, K.-A. A Comparative Study of Rutin and Rutin Glycoside: Antioxidant Activity, Anti-Inflammatory Effect, Effect on Platelet Aggregation and Blood Coagulation. Antioxidants 2021, 10, 1696. [Google Scholar]
- KFDA. Enzymatically modified hesperidin. Food Addit. Code 2021, 19, 329–330. [Google Scholar]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, L.; Duchnik, W.; Klimowicz, A. Enhancement of the antioxidant and skin permeation properties of eugenol by the esterification of eugenol to new derivatives. AMB Expr. 2020, 10, 187. [Google Scholar]
- Schroeder, P.; Klotz, L.O.; Sies, H. Amphiphilic properties of (−)-epicatechin and their significance for protection of cells against peroxynitrite. Biochem. Biophys. Res. Commun. 2003, 307, 69–73. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar]
- Ratha, P.; Jhon, D.Y. Increase of rutin, quercetin, and antioxidant activity during germinated buckwheat (Fagopyrum esculentum Moench) fermentation. Ferment. Technol. 2017, 6, 147. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substance. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [PubMed]
- Tagousop, C.; Tamokou, J.D.; Ekom, S.; Ngnokam, D.; Voutquenne, L. Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complement. Med. Ther. 2018, 18, 252. [Google Scholar]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar]
- Laskin, D.L.; Pendino, K.J. Macrophages and inflammatory mediators in tissue injury. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 655–677. [Google Scholar]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar]
- Wdowiak, K.; Walkowiak, J.; Pietrzak, R.; Bazan-Woźniak, A.; Cielecka-Piontek, J. Bioavailability of Hesperidin and Its Aglycone Hesperetin—Compounds Found in Citrus Fruits as a Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)—Mini-Review. Nutrients 2022, 14, 2647. [Google Scholar]
- Dolzhenko, Y.; Bertea, C.M.; Occhipinti, A.; Bossi, S.; Maffei, M.E. UV-B modulates the interplay between terpenoids and flavonoids in peppermint (Mentha × piperita L.). J. Photochem. Photobiol. B. 2010, 100, 67–75. [Google Scholar]
- Koh, J.S.; Koh, K.S.; Kim, Y.C. Changes of Some Flavonoids in the Peel of Late Maturing Citrus during Maturation. Prev. Nutr. Food Sci. 2002, 7, 1–4. [Google Scholar]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Sudheeran, P.K.; Ovadia, R.; Galsarker, O.; Maoz, I.; Sela, N.; Maurer, D.; Feygenberg, O.; Oren Shamir, M.; Alkan., N. Glycosylated flavonoids: Fruit’s concealed antifungal arsenal. New Phytol. 2020, 225, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hassan, Y.I.; Liu, R.; Mats, L.; Yang, C.; Liu, C.; Tsao, R. Molecular Mechanisms Underlying the Absorption of Aglycone and Glycosidic Flavonoids in a Caco-2 BBe1 Cell Model. ACS Omega 2020, 6, 10782–10793. [Google Scholar] [CrossRef] [PubMed]
- Srirangam, R.; Majumdar, S. Passive asymmetric transport of hesperetin across isolated rabbit cornea. Int. J. Pharm. 2010, 15, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S.; Tanabe, S.; Sugiyama, M.; Konishi, Y. Transepithelial transport of hesperetin and hesperidin in intestinal Caco-2 cell monolayers. Biochim. Biophys. Acta/Biomembr. 2008, 1778, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [CrossRef] [Green Version]
- Brett, G.M.; Hollands, W.; Needs, P.W.; Teucher, B.; Dainty, J.R.; Davis, B.D.; Brodbelt, J.S.; Kroon, P.A. Absorption, metabolism and excretion of flavanones from single portions of orange fruit and juice and effects of anthropometric variables and contraceptive pill use on flavanone excretion. Br. J. Nutr. 2009, 101, 664–675. [Google Scholar] [CrossRef] [Green Version]
- Borges, G.; Fong, R.Y.; Ensunsa, J.L.; Kimball, J.; Medici, V.; Ottaviani, J.I.; Crozier, A. Absorption, distribution, metabolism and excretion of apigenin and its glycosides in healthy male adults. Free Radic. Biol. Med. 2022, 20, 90–96. [Google Scholar] [CrossRef]
- Roowi, S.; Mullen, W.; Edwards, C.A.; Crozier, A. Yoghurt impacts on the excretion of phenolic acids derived from colonic breakdown of orange juice flavanones in humans. Mol. Nutr. Food Res. 2009, 53, 68–75. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Ludwig, I.A.; Polyviou, T.; Malkova, D.; Garcia, A.; Morenon-Rojas, J.M.; Crozier, A. Plasma and urinary metabolites and catabolites derived from orange juice (poly)phenols: Analysis by high performance liquid chromatography-high resolution-mass spectrometry. J. Agric. Food Chem. 2016, 64, 5724–5735. [Google Scholar] [CrossRef] [Green Version]
- Nectoux, A.M.; Abe, C.; Huang, S.W.; Ohno, N.; Tabata, J.; Miyata, Y.; Tanaka, K.; Tanaka, T.; Yamamura, H.; Matsui, T. Absorption and Metabolic Behavior of Hesperidin (Rutinosylated Hesperetin) after Single Oral Administration to Sprague-Dawley Rats. J. Agri. Food Chem. 2019, 67, 9812–9819. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Tanabe, F.; Arai, N.; Mitsuzumi, H.; Miwa, Y.; Kubota, M.; Chaen, H.; Kibata, M. Bioavailability of glucosyl hesperidin in rats. Biosci. Biotechnol. Biochem. 2006, 70, 1386–1394. [Google Scholar] [PubMed]
- Matsumoto, M.; Matsukawa, N.; Mineo, H.; Chiji, H.; Hara, H. A soluble flavonoid-glycoside, alpha G-rutin, is absorbed as glycosides in the isolated gastric and intestinal mucosa. Biosci. Biotechnol. Biochem. 2004, 68, 1929–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar]
- Ribeiro, C.B.; Ramos, F.M.; Manthey, J.A.; Cesar, T.B. Effectiveness of Eriomin® in managing hyperglycemia and reversal of prediabetes condition: A double-blind, randomized, controlled study. Phytother. Res. 2019, 33, 1921–1933. [Google Scholar] [PubMed] [Green Version]
- Yamada, M.; Mitsuzumi, H.; Tsuzaki, Y.; Miwa, Y.; Chaen, H.; Yamamoto, I. Antioxidant activity of glycosylated vitamin P and its suppressive effect on oxidative stress in hyperlipidemic mice. Jpn. Soc. Nutr. Food Sci. 2003, 56, 355–363. [Google Scholar]
- Johnson, J.B.; Mani, J.S.; Broszczak, D.; Prasad, S.S.; Ekanayake, C.P.; Strappe, P.; Valeris, P.; Naiker, M. Hitting the sweet spot: A systematic review of the bioactivity and health benefits of phenolic glycosides from medicinally used plants. Phytother. Res. 2021, 35, 3484–3508. [Google Scholar]
- Lesjak, M.; Beara, I.; Simin, N.; Pinta´c, D.; Majki´c, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Srirangam, R.; Hippalgaonkar, K.; Majumdar, S. Intravitreal kinetics of hesperidin, hesperetin, and hesperidin G: Effect of dose and physicochemical properties. J. Pharm. Sci. 2012, 101, 1631–1638. [Google Scholar]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure—Activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar]
- Kim, J.H.; Park, S.H.; Beak, E.J.; Han, C.H.; Kang, N.J. Anti-oxidant and anti-inflammatory effects of rutin and its metabolites. Curr. Res. Agric. Life Sci. 2013, 31, 165–169. [Google Scholar]
- Freedman, J.E. Oxidative stress and platelets. Arterioscler. Thromb. Vasc. Biol. 2008, 28, s11–s16. [Google Scholar] [PubMed] [Green Version]
- Zeng, Y.; Song, J.; Zhang, M.; Wang, H.; Zhang, Y.; Suo, H. Comparison of in vitro and in vivo antioxidant activities of six flavonoids with similar structures. Antioxidants 2020, 9, 732. [Google Scholar]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar]
- Habauzit, V.; Nielsen, I.; Gil-Izquierdo, A.; Trzeciakiewicz, A.; Morand, C.; Chee, W.; Barron, D.; Lebecque, P.; Davicco, M.J.; Williamson, G.; et al. Increased bioavailability of hesperetin-7-glucoside compared with hesperidin results in more efficient prevention of bone loss in adult ovariectomised rats. British J. Nutri. 2009, 102, 976–984. [Google Scholar]
- Sahiner, M.; Sahiner, N.; Sagbas, S.; Fullerton, M.L.; Blake, D.A. Fabrication of Biodegradable Poly(naringin) Particles with Antioxidant Activity and Low Toxicity. ACS Omega 2018, 3, 17359–17367. [Google Scholar]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar]
- Oztanir, M.N.; Ciftci, O.; Cetin, A.; Aladag, M.A. Hesperidin attenuates oxidative and neuronal damage caused by global cerebral ischemia/reperfusion in a C57BL/J6 mouse model. Neurol. Sci. 2014, 35, 1393–1399. [Google Scholar]
- Branchford, B.R.; Carpenter, S.L. The Role of Inflammation in Venous Thromboembolism. Front. Pediatr. 2018, 6, 142. [Google Scholar] [CrossRef] [PubMed]
- Donato, F.; de Gomes, M.G.; Goes, A.T.R.; Filho, C.B.; Del Fabbro, L.; Antunes, M.S.; Souza, L.C.; Boeira, S.P.; Jesse, C.R. Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: Possible role of l-arginine-NO-cGMP pathway and BDNF levels. Brain Res. Bull. 2014, 104, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, L.; Walzem, R.L.; Miller, E.G.; Pike, L.M.; Patil, B.S. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J. Agric. Food Chem. 2005, 53, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Vallejo, F.; Espín, J.C.; Tomás-Barberán, F.A. Hesperetin and its sulfate and glucuronide metabolites inhibit TNF-α induced human aortic endothelial cell migration and decrease plasminogen activator inhibitor-1 (PAI-1) levels. Food Funct. 2016, 7, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. Physiological Relevance of the Antiproliferative and Estrogenic Effects of Dietary Polyphenol Aglycones versus Their Phase-II Metabolites on Breast Cancer Cells: A Call of Caution. J. Agric. Food Chem. 2018, 15, 8547–8555. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar]
- Trivedi, P.P.; Tripathi, D.N.; Jena, G.B. Hesperetin protects testicular toxicity of doxorubicin in rat: Role of NFkappaB, p38 and caspase-3. Food Chem. Toxicol. 2011, 49, 838–847. [Google Scholar] [CrossRef]
- Iranshahi, M.; Rezaee, R.; Parhiz, H.; Roohbakhsh, A.; Soltani, F. Protective effects of flavonoids against microbes and toxins: The cases of hesperidin and hesperetin. Life Sci. 2015, 137, 125–132. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Han, S.S.; You, I.J. Studies on Antimicrobial Activities and Safety of Natural Naringin in Korea. Korean J. Mycol. 1988, 16, 33–40. [Google Scholar]
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, I.; Barboza, E.; Willig, G.; Marié, T.; Texeira, A.; Darme, P.; Renault, J.-H.; Allais, F. Implementation of an enzyme membrane reactor to intensify the α-O-glycosylation of resveratrol using cyclodextrins. Pharmaceuticals 2021, 14, 319. [Google Scholar] [CrossRef] [PubMed]








| Compound | Log Po/w |
|---|---|
| Hesperetin | 2.85 ± 0.05 a |
| Hesperidin Hesperidin mono-glucoside | 2.01 ± 0.02 b −3.04 ± 0.09 c |
| Microorganism | MIC Value (μg/mL) | ||
|---|---|---|---|
| Hesperetin | Hesperidin | Hesperidin Glucoside | |
| S. aureus (KCTC 3881) | 125 | 1000 | 500 |
| B.cereus (ATCC 21772) | 250 | 2000 | 500 |
| E. coli (KCTC 2571) | 250 | >2000 | 1000 |
| P. aeruginosa (KCTC 2513). | 500 | 2000 | 1000 |
| Microorganism | MBC Value (μg/mL) | ||
|---|---|---|---|
| Hesperetin | Hesperidin | Hesperidin Glucoside | |
| S. aureus (KCTC 3881) | 500 | >2000 | 1000 |
| B.cereus (ATCC 21772) | 500 | >2000 | 1000 |
| E. coli (KCTC 2571) | 1000 | >2000 | 2000 |
| P. aeruginosa (KCTC 2513). | 2000 | >2000 | 2000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. https://doi.org/10.3390/antiox11081618
Choi S-S, Lee S-H, Lee K-A. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants. 2022; 11(8):1618. https://doi.org/10.3390/antiox11081618
Chicago/Turabian StyleChoi, Sung-Sook, Sun-Hyung Lee, and Kyung-Ae Lee. 2022. "A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro" Antioxidants 11, no. 8: 1618. https://doi.org/10.3390/antiox11081618