Synthesis and Antioxidant Properties of HeteroBisNitrones Derived from Benzene Dicarbaldehydes
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.1.1. General Method for the Synthesis of hBNs 1–9
(Z)-N-tert-Butyl-1-(4-((Z)-(methyloxidoazaneylidene)methyl)phenyl)methanimine oxide (hBN1)
(Z)-N-Benzyl-1-(4-((Z)-methyloxidoazaneylidene)methyl)phenyl)methanimine oxide (hBN2)
(Z)-N-Benzyl-1-(4-((Z)-(tert-butyloxidoazaneylidene)methyl)phenyl)methanimine oxide (hBN3)
(Z)-N-tert-Butyl-1-(3-((Z)-(methyloxidoazaneylidene)methyl)phenyl)methanimine oxide (hBN4)
(Z)-N-Benzyl-1-(3-((Z)-methyloxidoazaneylidene)methyl)phenyl)methanimine oxide (hBN5)
(Z)-N-Benzyl-1-(3-((Z)-(tert-butyloxidoazaneylidene)methyl)phenyl)methanimine oxide (hBN6)
(Z)-N-tert-Butyl-1-(2-((Z)-(methyloxidoazaneylidene)methyl)phenyl)methanimine oxide (hBN7)
(Z)-N-Benzyl-1-(2-((Z)-(methyloxidoazaneylidene)methyl)phenyl)methanimine oxide (hBN8)
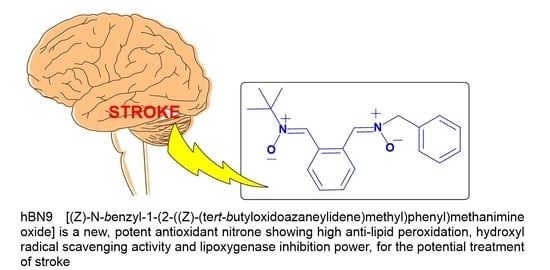
(Z)-N-Benzyl-1-(2-((Z)-(tert-butyloxidoazaneylidene)methyl)phenyl)methanimine oxide (hBN9)
2.2. Estimation of Lipophilicity as Clog P
2.3. Biological Antioxidant Assays Used for the Study of hBNs 1–9 and PBN
2.3.1. Inhibition of Linoleic Acid Peroxidation
2.3.2. In Vitro Inhibition of Soybean Lipoxygenase (LOX)
2.3.3. Competition of the Tested Compounds with DMSO for Hydroxyl Radicals
2.3.4. ABTS∙+–Decolorization Assay in Ethanolic Solution for Antioxidant Activity
3. Results and Discussion
3.1. Chemistry
3.2. Antioxidant Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Brouns, R.; De Deyn, P.P. The complexity of neurobiological processes in acute ischemic stroke. Clin. Neurol. Neurosurg. 2009, 111, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.S.; Lee, S.J.; Ryu, C.W.; Kim, H.S. Safety and efficacy of mechanical thrombectomy with solitaire stent retrieval for acute ischemic stroke: A systematic review. Neurointervention 2012, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nicole, O.; Docagne, F.; Ali, C.; Margaill, I.; Carmeliet, P.; MacKenzie, E.T.; Vivien, D.; Buisson, A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 2001, 7, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.M.; Georgiadis, A.L.; Vázquez, G.; Miley, J.T.; Memon, M.Z.; Mohammad, Y.M.; Christoforidis, G.A.; Tariq, N.; Qureshi, A.I. Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: A multicenter study. AJNR Am. J. Neuroradiol. 2010, 31, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.H. Cellular Antioxidant Defense Mechanisms; Chow, C.K., Ed.; CRC Press: Boca Ratón, FL, USA, 1988; Volume 3, pp. 89–109. [Google Scholar]
- Huang, Y.; Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Citron, M. Alzheimer’s disease: Strategies for disease modification. Nat. Rev. Drug Discov. 2010, 9, 387–398. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Esteban, G.; Brogi, S.; Shionoya, M.; Wang, L.; Campiani, G.; Unzeta, M.; Inokuchi, T.; Stefania Butini, S.; Marco-Contelles, J. Donepezi-l-like multifunctional agents: Design, synthesis, molecular modeling and biological evaluation. Eur. J. Med. Chem. 2016, 121, 864–879. [Google Scholar] [CrossRef]
- Alonso, J.M.; Escobar-Peso, A.; Palomino-Antolín, A.; Diez-Iriepa, D.; Chioua, M.; Martínez-Alonso, E.; Iriepa, I.; Egea, J.; Alcázar, J.; Marco-Contelles, J. Privileged quinolylnitrones for the combined therapy of ischemic stroke and Alzheimer’s disease. Pharmaceuticals 2021, 14, 861. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.P.; Angiolini, P.; Tani, R.; Consales, G.; Bordi, L. Phenyl-t-butyl-nitrone is active against traumatic shock in rats. Free Radic. Res. Commun. 1986, 1, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Deletraz, A.; Zéamari, K.; Hua, K.; Combes, M.; Villamena, F.A.; Tuccio, B.; Callizot, N.; Durand, G. Substituted α-phenyl and α-naphthlyl-N-tert-butyl nitrones: Synthesis, spin-trapping, and neuroprotection evaluation. J. Org. Chem. 2020, 85, 6073–6085. [Google Scholar] [CrossRef] [PubMed]
- Marco-Contelles, J. Recent advances on nitrones design for stroke treatment. J. Med. Chem. 2020, 63, 13413–13427. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.A.; Ley, J.J.; Echegoyen, L.; Alvarado, R. Stilbazulenyl nitrone (STAZN): A nitronyl-substituted hydrocarbon with the potency of classical phenolic chain-breaking antioxidants. J. Am. Chem. Soc. 2002, 124, 4678–4684. [Google Scholar] [CrossRef] [PubMed]
- Althaus, J.S.; Fleck, T.J.; Becker, D.A.; Hall, E.D.; Von Voigtlander, P.F. Azulenyl nitrones: Colorimetric detection of oxyradical end products and neuroprotection in the gerbil transient forebrain ischemia/reperfusion model. Free Radical Biol. Med. 1998, 24, 738–744. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, G.; Zhang, Z.; Yu, P.; Zhong, H.; Du, J.; Wang, Y. Novel multi-functional nitrones for treatment of ischemic stroke. Bioorg. Med. Chem. 2012, 20, 3939–3945. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, B.; Diez-Iriepa, D.; Merás-Sáiz, B.; Chioua, M.; García-Vieira, D.; Iriepa, I.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Martínez-Murillo, R.; González-Nieto, D.; et al. Synthesis, antioxidant properties and neuroprotection of α-phenyl-tert-butylnitrone derived homobisnitrones in in vitro and in vivo ischemia models. Sci. Rep. 2020, 10, 14150. [Google Scholar] [CrossRef] [PubMed]



| Nitrones/ Standard | ClogP b | ILPO (%) | LOX Inhibition (% or IC50 [μM]) | Scav. Activity for •OH (%) | ABTS+. (%) |
|---|---|---|---|---|---|
| PBN | 3.02 | 11 | 23 | no | 5 |
| HBN6 [18] | 4.96 | 37 | 29 | 81 | No |
| hBN1 | 0.04 | 43 | 11 | 43 | no |
| hBN2 | 0.69 | 48 | nο | 79 | no |
| hBN3 | 1.93 | 60 | 11 | 69 | 5 |
| hBN4 | 0.04 | no | no | 91 | 11 |
| hBN5 | 0.57 | no | no | 96.8 | no |
| hBN6 | 1.80 | no | 16 | 68.3 | no |
| hBN7 | 0.04 | 41.5 | 10 | 100 | 11.6 |
| hBN8 | 0.57 | no | 82.5 μM | 89 | 7.6 |
| hBN9 | 1.80 | 100 | 57.5 μM | 99.8 | 11.2 |
| NDGA | 3.92 | 0.45 μM | |||
| Trolox | 3.09 | 93 | 73 | 91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diez-Iriepa, D.; Iriepa, I.; López-Muñoz, F.; Marco-Contelles, J.; Hadjipavlou-Litina, D. Synthesis and Antioxidant Properties of HeteroBisNitrones Derived from Benzene Dicarbaldehydes. Antioxidants 2022, 11, 1575. https://doi.org/10.3390/antiox11081575
Diez-Iriepa D, Iriepa I, López-Muñoz F, Marco-Contelles J, Hadjipavlou-Litina D. Synthesis and Antioxidant Properties of HeteroBisNitrones Derived from Benzene Dicarbaldehydes. Antioxidants. 2022; 11(8):1575. https://doi.org/10.3390/antiox11081575
Chicago/Turabian StyleDiez-Iriepa, Daniel, Isabel Iriepa, Francisco López-Muñoz, José Marco-Contelles, and Dimitra Hadjipavlou-Litina. 2022. "Synthesis and Antioxidant Properties of HeteroBisNitrones Derived from Benzene Dicarbaldehydes" Antioxidants 11, no. 8: 1575. https://doi.org/10.3390/antiox11081575