Hydrogen Sulfide, Ethylene, and Nitric Oxide Regulate Redox Homeostasis and Protect Photosynthetic Metabolism under High Temperature Stress in Rice Plants
Abstract
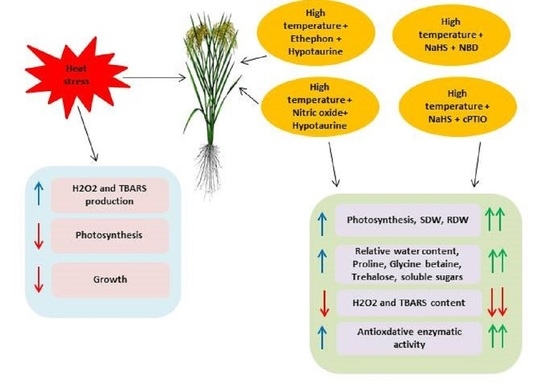
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Experimental Design
2.2. Measurement of Photosynthetic and Growth Characteristics
2.3. Chlorophyll Fluorescence Measurement
2.4. Leaf Relative Water Content (RWC) Determination
2.5. Estimation of the Contents of Proline, Glycine Betaine (GB), Trehalose and Soluble Sugars
2.6. Measurement of Hydrogen Peroxide (H2O2) and Lipid Peroxidation
2.7. Assay of Antioxidant Enzymes Activities
2.8. Determination of Nitric Oxide, Hydrogen Sulfide, and Ethylene
2.9. RNA Isolation and cDNA Synthesis
2.10. Quantitative Real-Time PCR Analysis
2.11. Statistical Analysis
3. Results
3.1. Growth Parameters
3.2. Gas-Exchange Parameters and Chlorophyll Content
3.3. Chlorophyll Fluorescence Parameters
3.4. Leaf RWC
3.5. Accumulation of Osmolytes
3.6. Oxidative Stress
3.7. Antioxidants Enzyme Activity
3.8. Hydrogen Sulfide and NO Content and Ethylene Production
3.9. Expression of Photosynthesis-Related Genes and Genes Encoding Antioxidant Enzymes
3.10. Determination of Interaction among Morpho-Physiological, Biochemical and Molecular Parameters through PCA
3.11. Pearson Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Yang, H.; Ding, L.; Song, Z.T.; Ma, H.; Chang, F.; Liu, J.X. Tissue-specific transcriptomics reveals an important role of the unfolded protein response in maintaining fertility upon heat stress in Arabidopsis. Plant Cell 2017, 29, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl jasmonate protects the PS II system by maintaining the stability of chloroplast d1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Gautam, H.; Sehar, Z.; Rehman, M.T.; Hussain, A.; AlAjmi, M.F.; Khan, N.A. Nitric oxide enhances photosynthetic nitrogen and sulfur-use efficiency and activity of ascorbate-glutathione cycle to reduce high temperature stress stress-induced oxidative stress in rice (Oryza sativa L.) plants. Biomolecules 2021, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Janská, A.; Maršík, P.; Zelenková, S.; Ovesná, J. Cold stress and acclimation—What is important for metabolic adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Nitric oxide and hydrogen sulfide coordinately reduce glucose sensitivity and decrease oxidative stress via ascorbate-glutathione cycle in heat-stressed wheat (Triticum aestivum L.) plants. Antioxidants 2021, 10, 108. [Google Scholar] [CrossRef]
- Jha, U.C.; Nayyar, H.; Palakurthi, R.; Jha, R.; Valluri, V.; Bajaj, P.; Thudi, M. Major QTLs and potential candidate genes for heat stress tolerance identified in chickpea (Cicer arietinum L.). Front. Plant Sci. 2021, 12, 655103. [Google Scholar] [CrossRef]
- Sehar, Z.; Iqbal, N.; Khan, M.I.R.; Masood, A.; Rehman, M.T.; Hussain, A.; Khan, N.A. Ethylene reduces glucose sensitivity and reverses photosynthetic repression through optimization of glutathione production in salt-stressed wheat (Triticum aestivum L.). Sci. Rep. 2021, 11, 12650. [Google Scholar] [CrossRef]
- Shivaraj, S.M.; Vats, S.; Bhat, J.A.; Dhakte, P.; Goyal, V.; Khatri, P.; Deshmukh, R. Nitric oxide and hydrogen sulfide crosstalk during heavy metal stress in plants. Physiol. Plant. 2020, 168, 437–455. [Google Scholar] [CrossRef]
- Iqbal, N.; Fatma, M.; Gautam, H.; Umar, S.; Sofo, A.; D’Ippolito, I.; Khan, N.A. The crosstalk of melatonin and hydrogen sulfide determines photosynthetic performance by regulation of carbohydrate metabolism in wheat under heat stress. Plants 2021, 10, 1778. [Google Scholar] [CrossRef]
- Fatma, M.; Iqbal, N.; Gautam, H.; Sehar, Z.; Sofo, A.; D’Ippolito, I.; Khan, N.A. Ethylene and sulfur coordinately modulate the antioxidant system and ABA accumulation in mustard plants under salt stress. Plants 2021, 10, 180. [Google Scholar] [CrossRef]
- Gautam, H.; Sehar, Z.; Khan, N.A. Ethylene: A small signaling molecule with diverse roles. In The Plant Hormone Ethylene. Stress Acclimation and Agricultural Applications; Khan, N.A., Ferrante, A., Munne-Bosch, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–10. [Google Scholar]
- Jegadeesan, S.; Chaturvedi, P.; Ghatak, A.; Pressman, E.; Meir, S.; Faigenboim, A.; Firon, N. Proteomics of heat-stress and ethylene-mediated thermotolerance mechanisms in tomato pollen grains. Front. Plant Sci. 2018, 9, 1558. [Google Scholar] [CrossRef]
- Poór, P.; Nawaz, K.; Gupta, R.; Ashfaque, F.; Khan, M.I.R. Ethylene involvement in the regulation of heat stress tolerance in plants. Plant Cell Rep. 2021, 41, 675–698. [Google Scholar] [CrossRef]
- Singh, G.; Sarkar, N.K.; Grover, A. Tango between ethylene and HSFA2 settles heat tolerance. Trends Plant Sci. 2021, 26, 429–432. [Google Scholar] [CrossRef]
- Wu, Y.S.; Yang, C.Y. Ethylene-mediated signaling confers thermotolerance and regulates transcript levels of heat shock factors in rice seedlings under heat stress. Bot. Stud. 2019, 60, 23. [Google Scholar] [CrossRef]
- Chen, K.; Chen, L.; Fan, J.; Fu, J. Alleviation of heat damage to photosystem II by nitric oxide in tall fescue. Photosynth. Res. 2013, 116, 21–31. [Google Scholar] [CrossRef]
- Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A. Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front. Plant Sci. 2016, 7, 521. [Google Scholar] [CrossRef]
- Parankusam, S.; Adimulam, S.S.; Bhatnagar-Mathur, P.; Sharma, K.K. Nitric oxide (NO) in plant heat stress tolerance: Current knowledge and perspectives. Front. Plant Sci. 2017, 8, 1582. [Google Scholar] [CrossRef]
- Rather, B.A.; Masood, A.; Sehar, Z.; Majid, A.; Anjum, N.A.; Khan, N.A. Mechanisms and role of nitric oxide in phytotoxicity-mitigation of copper. Front. Plant Sci. 2020, 11, 675. [Google Scholar] [CrossRef]
- Xuan, Y.; Zhou, S.; Wang, L.; Cheng, Y.; Zhao, L. Nitric oxide functions as a signal and acts upstream of AtCaM3 in thermotolerance in Arabidopsis seedlings. Plant Physiol. 2010, 153, 1895–1906. [Google Scholar] [CrossRef]
- Rather, B.A.; Mir, I.R.; Masood, A.; Anjum, N.A.; Khan, N.A. Nitric oxide pre-treatment advances seed germination and alleviates copper-induced photosynthetic inhibition in Indian mustard. Plants 2020, 9, 776. [Google Scholar] [CrossRef]
- Graziano, M.; Beligni, M.V.; Lamattina, L. Nitric oxide improves internal iron availability in plants. Plant Physiol. 2002, 130, 1852–1859. [Google Scholar] [CrossRef]
- Vani, B.; Saradhi, P.P.; Mohanty, P. Alteration in chloroplast structure and thylakoid membrane composition due to in vivo heat treatment of rice seedlings: Correlation with the functional changes. J. Plant Physiol. 2001, 158, 583–592. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vicente, I.; Lorenzo, O. Nitric oxide regulation of temperature acclimation: A molecular genetic perspective. J. Exp. Bot. 2021, 72, 5789–5794. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; González-Gordo, S.; Cañas, A.; Palma, J.M. Nitric oxide and hydrogen sulfide in plants: Which comes first? J. Exp. Bot. 2019, 70, 4391–4404. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.; Yruela, I.; Gotor, C.; Bassham, D.C. Persulfidation of ATG18a regulates autophagy under ER stress in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2023604118. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Jia, H.; Li, F.; Ma, Y.; Liesche, J.; Li, J. Persulfidation-induced structural change in SnRK2. 6 establishes intramolecular interaction between phosphorylation and persulfidation. Mol. Plant 2021, 14, 1814–1830. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Xie, Y. Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integr. Plant Biol. 2021, 63, 146–160. [Google Scholar] [CrossRef]
- Papanatsiou, M.; Scuffi, D.; Blatt, M.R.; García-Mata, C. Hydrogen sulfide regulates inward-rectifying K+ channels in conjunction with stomatal closure. Plant Physiol. 2015, 168, 29–35. [Google Scholar] [CrossRef]
- Pandey, A.K.; Gautam, A. Stress responsive gene regulation in relation to hydrogen sulfide in plants under abiotic stress. Physiol. Plant. 2020, 168, 511–525. [Google Scholar] [CrossRef]
- Huang, D.; Huo, J.; Liao, W. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2021, 302, 110733. [Google Scholar] [CrossRef]
- Li, H.; Yu, T.T.; Ning, Y.S.; Li, H.; Zhang, W.W.; Yang, H.Q. Hydrogen Sulfide Alleviates Alkaline Salt Stress by Regulating the Expression of MicroRNAs in Malus hupehensis Rehd. Roots. Front. Plant Sci. 2021, 12, 663519. [Google Scholar] [CrossRef]
- Min, Y.A.N.G.; Qin, B.P.; Ping, W.A.N.G.; Li, M.L.; Chen, L.L.; Chen, L.T.; Yin, Y.P. Foliar application of sodium hydrosulfide (NaHS), a hydrogen sulfide (H2S) donor, can protect seedlings against heat stress in wheat (Triticum aestivum L.). J. Integr. Agric. 2016, 15, 2745–2758. [Google Scholar]
- Husain, T.; Suhel, M.; Prasad, S.M.; Singh, V.P. Ethylene needs endogenous hydrogen sulfide for alleviating hexavalent chromium stress in Vigna mungo L. and Vigna radiata L. Environ. Pollut. 2021, 290, 117968. [Google Scholar] [CrossRef]
- Jia, H.; Chen, S.; Liu, D.; Liesche, J.; Shi, C.; Wang, J.; Li, J. Ethylene-induced hydrogen sulfide negatively regulates ethylene biosynthesis by persulfidation of ACO in tomato under osmotic stress. Front. Plant Sci. 2018, 9, 1517. [Google Scholar] [CrossRef]
- Yao, G.F.; Wei, Z.Z.; Li, T.T.; Tang, J.; Huang, Z.Q.; Yang, F.; Zhang, H. Modulation of enhanced antioxidant activity by hydrogen sulfide antagonization of ethylene in tomato fruit ripening. J. Agric. Food Chem. 2018, 66, 10380–10387. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A. Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma 2014, 251, 1007–1019. [Google Scholar] [CrossRef]
- Yang, S. Ethylene evolution from 2-chloroethylphosphonic acid. Plant Physiol. 1969, 44, 1203. [Google Scholar] [CrossRef]
- Biddle, E.; Kerfoot, D.G.; Kho, Y.H.; Russell, K.E. Kinetic studies of the thermal decomposition of 2-chloroethylphosphonic acid in aqueous solution. Plant Physiol. 1976, 58, 700–702. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Trevelyan, W.E.; Harrison, J. Studies on yeast metabolism. 5. The trehalose content of baker’s yeast during anaerobic fermentation. Biochem. J. 1956, 62, 177. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant 2015, 37, 9. [Google Scholar] [CrossRef]
- Okuda, T.; Matsuda, Y.; Yamanaka, A.; Sagisaka, S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef]
- Gautam, H.; Fatma, M.; Sehar, Z.; Iqbal, N.; Albaqami, M.; Khan, N.A. Exogenously-Sourced Ethylene Positively Modulates Photosynthesis, Carbohydrate Metabolism, and Antioxidant Defense to Enhance Heat Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 1031. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Beyer Jr, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Guo, Z.; Xing, J.; Huang, B. Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J. Exp. Bot. 2005, 56, 3223–3228. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, C.; Lai, D.; Sun, Y.; Samma, M.K.; Zhang, J.; Shen, W. Hydrogen sulfide delays GA-triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J. Plant Physiol. 2014, 171, 53–62. [Google Scholar] [CrossRef]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid responses of plants to temperature changes. Temperature 2017, 4, 371–405. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Zanganeh, R.; Jamei, R.; Rahmani, F. Role of salicylic acid and hydrogen sulfide in promoting lead stress tolerance and regulating free amino acid composition in Zea mays L. Acta Physiol. Plant. 2019, 41, 94. [Google Scholar] [CrossRef]
- Rizwan, M.; Mostofa, M.G.; Ahmad, M.Z.; Zhou, Y.; Adeel, M.; Mehmood, S.; Liu, Y. Hydrogen sulfide enhances rice tolerance to nickel through the prevention of chloroplast damage and the improvement of nitrogen metabolism under excessive nickel. Plant Physiol. Biochem. 2019, 138, 100–111. [Google Scholar] [CrossRef]
- Valivand, M.; Amooaghaie, R.; Ahadi, A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol. Biochem. 2019, 143, 286–298. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Pazmino, D.M.; Testillano, P.S.; Risueño, M.C.; Del Río, L.A.; Sandalio, L.M. Cellular response of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009, 150, 229–243. [Google Scholar] [CrossRef]
- Louis, X.L.; Murphy, R.; Thandapilly, S.J.; Yu, L.; Netticadan, T. Garlic extracts prevent oxidative stress, hypertrophy and apoptosis in cardiomyocytes: A role for nitric oxide and hydrogen sulfide. BMC Complement. Altern. Med. 2012, 12, 140. [Google Scholar] [CrossRef]
- Li, Z.G.; Yang, S.Z.; Long, W.B.; Yang, G.X.; Shen, Z.Z. Hydrogen sulphide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. 2013, 36, 1564–1572. [Google Scholar] [CrossRef]
- Sehar, Z.; Gautam, H.; Iqbal, N.; Alvi, A.F.; Jahan, B.; Fatma, M.; Albaqami, M.; Khan, N.A. The functional interplay between ethylene, hydrogen sulfide, and sulfur in plant heat stress tolerance. Biomolecules 2022, 12, 678. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, Y.; Chi, Y.; Zhou, L.; Chen, J.; Zhou, W.; Ding, J. Drought stress strengthens the link between chlorophyll fluorescence parameters and photosynthetic traits. PeerJ 2020, 8, e10046. [Google Scholar] [CrossRef]
- Lu, Y. Identification and roles of photosystem II assembly, stability, and repair factors in Arabidopsis. Front. Plant Sci. 2016, 7, 168. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Havaux, M. Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant Cell Environ. 1993, 16, 461–467. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature stress effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Yamamoto, Y. Quality control of photosystem II: The mechanisms for avoidance and tolerance of light and heat stresses are closely linked to membrane fluidity of the thylakoids. Front. Plant Sci. 2016, 7, 1136. [Google Scholar] [CrossRef] [PubMed]
- Marutani, Y.; Yamauchi, Y.; Kimura, Y.; Mizutani, M.; Sugimoto, Y. Damage to photosystem II due to heat stress without light-driven electron flow: Involvement of enhanced introduction of reducing power into thylakoid membranes. Planta 2012, 236, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Seo, C.W.; Khan, A.L.; Mun, B.G.; Shahzad, R.; Ko, J.W.; Lee, I.J. Exo-ethylene application mitigates waterlogging stress in soybean (Glycine max L.). BMC Plant Biol. 2018, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, G.; Wang, Y.; Zhang, L.; Zhang, L. Protective effect of nitric oxide against oxidative stress under ultraviolet-B radiation. Nitric Oxide 2005, 13, 1–9. [Google Scholar] [CrossRef]
- Morales, D.; Rodríguez, P.; Dell’Amico, J.; Nicolas, E.; Torrecillas, A.; Sánchez-Blanco, M.J. high temperature stress preconditioning and thermal shock imposition affects water relations, gas exchange and root hydraulic conductivity in tomato. Biol. Plant. 2003, 47, 203–208. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 2012, 27, 210–218. [Google Scholar] [CrossRef]
- Li, G.; Shah, A.A.; Khan, W.U.; Yasin, N.A.; Ahmad, A.; Abbas, M.; Safdar, N. Hydrogen sulfide mitigates cadmium induced toxicity in Brassica rapa by modulating physiochemical attributes, osmolyte metabolism and antioxidative machinery. Chemosphere 2021, 263, 127999. [Google Scholar] [CrossRef]
- Duan, B.; Ma, Y.; Jiang, M.; Yang, F.; Ni, L.; Lu, W. Improvement of photosynthesis in rice (Oryza sativa L.) as a result of an increase in stomatal aperture and density by exogenous hydrogen sulfide treatment. Plant Growth Regul. 2015, 75, 33–44. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B.J.B.P. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot. Stud. 2013, 54, 45. [Google Scholar] [CrossRef]
- Yang, X.; Wen, X.; Gong, H.; Lu, Q.; Yang, Z.; Tang, Y.; Lu, C. Genetic engineering of the biosynthesis of glycinebetaine enhances thermotolerance of photosystem II in tobacco plants. Planta 2007, 225, 719–733. [Google Scholar] [CrossRef]
- Rizvi, A.; Khan, M.S. Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicol. Environ. Saf. 2018, 157, 9–20. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Carbohydrate accumulation in relation to heat stress tolerance in two creeping bentgrass cultivars. J. Am. Soc. Hortic. Sci. 2000, 125, 442–447. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Guy, C.L. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef]
- Luo, Y.; Li, F.; Wang, G.P.; Yang, X.H.; Wang, W. Exogenously-supplied trehalose protects thylakoid membranes of winter wheat from heat-induced damage. Biol. Plant. 2010, 54, 495–501. [Google Scholar] [CrossRef]
- Kosar, F.; Akram, N.A.; Sadiq, M.; Al-Qurainy, F.; Ashraf, M. Trehalose: A key organic osmolyte effectively involved in plant abiotic stress tolerance. J. Plant Growth Regul. 2019, 38, 606–618. [Google Scholar] [CrossRef]
- Li, Z.G.; Luo, L.J.; Zhu, L.P. Involvement of trehalose in hydrogen sulfide donor sodium hydrosulfide-induced the acquisition of heat tolerance in maize (Zea mays L.) seedlings. Bot. Stud. 2014, 55, 20. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Siddiqui, M.H. Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide 2017, 68, 91–102. [Google Scholar] [CrossRef]
- Iqbal, N.; Sehar, Z.; Fatma, M.; Umar, S.; Sofo, A.; Khan, N.A. Nitric Oxide and Abscisic Acid Mediate Heat Stress Tolerance through Regulation of Osmolytes and Antioxidants to Protect Photosynthesis and Growth in Wheat Plants. Antioxidants 2022, 11, 372. [Google Scholar] [CrossRef]
- Kong, W.W.; Huang, C.Y.; Chen, Q.; Zou, Y.J.; Zhao, M.R.; Zhang, J.X. Nitric oxide is involved in the regulation of trehalose accumulation under heat stress in Pleurotus eryngii var. tuoliensis. Biotechnol. Lett. 2012, 34, 1915–1919. [Google Scholar] [CrossRef]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 2015, 3, 11. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Chan, Z. Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol. Biochem. 2013, 71, 226–234. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Khan, N.A. Ethephon mitigates nickel stress by modulating antioxidant system, glyoxalase system and proline metabolism in Indian mustard. Physiol. Mol. Biol. Plants 2020, 26, 1201–1213. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Chan, Z. Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol. Biochem. 2014, 74, 99–107. [Google Scholar] [CrossRef]
- Peng, R.; Bian, Z.; Zhou, L.; Cheng, W.; Hai, N.; Yang, C.; Wang, C. Hydrogen sulfide enhances nitric oxide-induced tolerance of hypoxia in maize (Zea mays L.). Plant Cell Rep. 2016, 35, 2325–2340. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, S.; Kumar, J.; Prasad, S.M. Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate–glutathione cycle: Possible involvement of nitric oxide. J. Plant Physiol. 2015, 181, 20–29. [Google Scholar] [CrossRef]
- Per, T.S.; Masood, A.; Khan, N.A. Nitric oxide improves S-assimilation and GSH production to prevent inhibitory effects of cadmium stress on photosynthesis in mustard (Brassica juncea L.). Nitric Oxide 2017, 68, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.P.; Xing, Y.; Zhang, J.H. Role of nitric oxide dependence on nitric oxide synthase-like activity in the water stress signaling of maize seedling. J. Integr. Plant Biol. 2008, 50, 435–442. [Google Scholar] [CrossRef]
- Zhao, M.G.; Chen, L.; Zhang, L.L.; Zhang, W.H. Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol. 2009, 151, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.J.; Zhang, S.L.; Li, D.F.; Zhao, Y.Z.; Tian, X.L.; Zhao, X.L.; Liu, R.Q. Effects of exogenous hydrogen sulfide on the ascorbate and glutathione metabolism in wheat seedlings leaves under water stress. Acta Physiol. Plant. 2011, 33, 2533. [Google Scholar] [CrossRef]
- Ziogas, V.; Tanou, G.; Filippou, P.; Diamantidis, G.; Vasilakakis, M.; Fotopoulos, V.; Molassiotis, A. Nitrosative responses in citrus plants exposed to six abiotic stress conditions. Plant Physiol. Biochem. 2013, 68, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, V.; Tanou, G.; Belghazi, M.; Filippou, P.; Fotopoulos, V.; Grigorios, D.; Molassiotis, A. Roles of sodium hydrosulfide and sodium nitroprusside as priming molecules during drought acclimation in citrus plants. Plant Mol. Biol. 2015, 89, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Abozeid, A.; Ying, Z.; Lin, Y.; Liu, J.; Zhang, Z.; Tang, Z. Ethylene improves root system development under cadmium stress by modulating superoxide anion concentration in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 253. [Google Scholar] [CrossRef]
- Cao, S.; Jiang, S.; Zhang, R. Evidence for a role of Ethylene-Insensitive 2 gene in the regulation of the oxidative stress response in Arabidopsis. Acta Physiol. Plant. 2006, 28, 417–425. [Google Scholar] [CrossRef]
- Cao, Y.; Song, F.; Goodman, R.M.; Zheng, Z. Molecular characterization of four rice genes encoding ethylene-responsive transcriptional factors and their expressions in response to biotic and abiotic stress. J. Plant Physiol. 2006, 163, 1167–1178. [Google Scholar] [CrossRef]
- Li, C.H.; Wang, G.; Zhao, J.L.; Zhang, L.Q.; Ai, L.F.; Han, Y.F.; Sun, Y. The receptor-like kinase SIT1 mediates salt sensitivity by activating MAPK3/6 and regulating ethylene homeostasis in rice. Plant Cell 2014, 26, 2538–2553. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Sidhu, G.P.S.; Kumar, R.; Kohli, S.K.; Yadav, P.; Bhardwaj, R. Abiotic stress management in plants: Role of ethylene. In Molecular Plant Abiotic Stress: Biology and Biotechnology; Wiley: London, UK, 2019; pp. 185–208. [Google Scholar]
- Neill, S.; Barros, R.; Bright, J.; Desikan, R.; Hancock, J.; Harrison, J.; Wilson, I. Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 2008, 59, 165–176. [Google Scholar] [CrossRef]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; López-Jaramillo, J.; Barroso, J.B. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. J. Exp. Bot. 2014, 65, 527–538. [Google Scholar] [CrossRef]
- Clark, D.; Durner, J.; Navarre, D.A.; Klessig, D.F. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol. Plant Microbe Interact. 2000, 13, 1380–1384. [Google Scholar] [CrossRef]
- Christou, A.; Filippou, P.; Manganaris, G.A.; Fotopoulos, V. Sodium hydrosulfide induces systemic thermotolerance to strawberry plants through transcriptional regulation of heat shock proteins and aquaporin. BMC Plant Biol. 2014, 14, 42. [Google Scholar] [CrossRef]
- Locato, V.; Gadaleta, C.; De Gara, L.; De Pinto, M.C. Production of reactive species and modulation of antioxidant network in response to heat shock: A critical balance for cell fate. Plant Cell Environ. 2008, 31, 1606–1619. [Google Scholar] [CrossRef]
- Ya’acov, Y.L.; Wills, R.B.; Ku, V.V.V. Evidence for the function of the free radical gas–nitric oxide (NO•)—As an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol. Biochem. 1998, 36, 825–833. [Google Scholar]
- Li, Z.G.; Yi, X.Y.; Li, Y.T. Effect of pretreatment with hydrogen sulfide donor sodium hydrosulfide on heat tolerance in relation to antioxidant system in maize (Zea mays) seedlings. Biol. Plant. 2014, 69, 1001–1009. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.H.; Wu, F.H.; He, E.M.; Liu, X.; Shangguan, Z.P.; Zheng, H.L. Hydrogen sulfide enhances salt tolerance through nitric oxide-mediated maintenance of ion homeostasis in barley seedling roots. Sci. Rep. 2015, 5, 12516. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant. 2020, 168, 345–360. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; He, L.F. Role of nitric oxide and hydrogen sulfide in plant aluminum tolerance. Biometals 2019, 32, 1–9. [Google Scholar] [CrossRef]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Sauter, K.J.; Davis, D.W.; Li, P.H.; Wallerstein, I.S. Leaf ethylene evolution level following high temperature stress in common bean. Hort. Sci. 1990, 25, 1282–1284. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Per, T.S.; Khan, N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013, 8, e26374. [Google Scholar] [CrossRef]
- Tao, J.J.; Chen, H.W.; Ma, B.; Zhang, W.K.; Chen, S.Y.; Zhang, J.S. The role of ethylene in plants under salinity stress. Front. Plant Sci. 2015, 6, 1059. [Google Scholar] [CrossRef]
- Steams, J.C.; Glick, B.R. Transgenic plants with altered ethylene biosynthesis or perception. Biotechnol. Adv. 2003, 21, 193–210. [Google Scholar]
- Manjunatha, G.; Gupta, K.J.; Lokesh, V.; Mur, L.A.; Neelwarne, B. Nitric oxide counters ethylene effects on ripening fruits. Plant Signal. Behav. 2012, 7, 476–483. [Google Scholar] [CrossRef]
- Ge, Y.; Hu, K.D.; Wang, S.S.; Hu, L.Y.; Chen, X.Y.; Li, Y.H.; Zhang, H. Hydrogen sulfide alleviates postharvest ripening and senescence of banana by antagonizing the effect of ethylene. PLoS ONE 2017, 12, e0180113. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, J.; Liu, T.; Xue, S. Hydrogen sulfide (H2S) signaling in plant development and stress responses. Abiotech 2021, 2, 32–63. [Google Scholar] [CrossRef]
- Lípová, L.; Krchňák, P.; Komenda, J.; Ilík, P. Heat-induced disassembly and degradation of chlorophyll-containing protein complexes in vivo. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 63–70. [Google Scholar] [CrossRef]
- Mulo, P.; Sakurai, I.; Aro, E.M. Strategies for psbA gene expression in cyanobacteria, green algae and higher plants: From transcription to PSII repair. Biochim. Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 247–257. [Google Scholar] [CrossRef]
- Barber, J.; Nield, J.; Morris, E.P.; Zheleva, D.; Hankamer, B. The structure, function and dynamics of photosystem two. Physiol. Plant. 1997, 100, 817–827. [Google Scholar] [CrossRef]
- Hassan, H.; Alatawi, A.; Abdulmajeed, A.; Emam, M.; Khattab, H. Roles of Si and SiNPs in Improving Thermotolerance of Wheat Photosynthetic Machinery via Upregulation of PsbH, PsbB and PsbD Genes Encoding PSII Core Proteins. Sci. Hortic. 2021, 7, 16. [Google Scholar] [CrossRef]
- Varghese, N.; Alyammahi, O.; Nasreddine, S.; Alhassani, A.; Gururani, M.A. Melatonin positively influences the photosynthetic machinery and antioxidant system of Avena sativa during salinity stress. Plants 2019, 8, 610. [Google Scholar] [CrossRef]
- Aziz, A.; Mahmood, T.; Mahmood, Z.; Shazadi, K.; Mujeeb-Kazi, A.; Rasheed, A. Genotypic variation and genotype × environment interaction for yield-related traits in synthetic hexaploid wheats under a range of optimal and heat-stressed environments. Crop Sci. 2018, 58, 295–303. [Google Scholar] [CrossRef]
- Ali, Q.; Perveen, R.; El-Esawi, M.A.; Ali, S.; Hussain, S.M.; Amber, M.; Ahmad, P. Low doses of Cuscuta reflexa extract act as natural biostimulants to improve the germination vigor, growth, and grain yield of wheat grown under water stress: Photosynthetic pigments, antioxidative defense mechanisms, and nutrient acquisition. Biomolecules 2020, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Basit, F.; Chen, M.; Ahmed, T.; Shahid, M.; Noman, M.; Liu, J.; Guan, Y. Seed priming with brassinosteroids alleviates chromium stress in rice cultivars via improving ROS metabolism and antioxidant defense response at biochemical and molecular levels. Antioxidants 2021, 10, 1089. [Google Scholar] [CrossRef] [PubMed]
- Sneath, P.H.; Sokal, R.R. Numerical Taxonomy: The Principles and Practice of Numerical Classification; W. H. Freeman and Company: San Francisco, CA, USA, 1973; p. 573. [Google Scholar]








| Taipei-309 | Rasi | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | Net Photosynthetic Rate | Stomatal Conductance | Intercellular CO2 Concentration | SPAD | Net Photosynthetic Rate | Stomatal Conductance | Intercellular CO2 Concentration | SPAD |
| Control | 15.1 ± 1.08 defg | 367.2 ± 15.0 de | 260.1 ± 13.8 de | 33.7 ± 1.30 g | 14.4 ± 1.04 fgh | 355.3 ± 14.0 ef | 251.2 ± 12.4 ef | 31.5 ± 1.25 gh |
| HS | 9.8 ± 0.66 ij | 268.3 ± 12.2 gh | 199.8 ± 11.6 gh | 24.1 ± 1.10 kl | 9.2 ± 0.55 j | 254.8 ± 11.0 h | 190.3 ± 10.2 h | 22.3 ± 1.0 l |
| Eth | 21.7 ± 1.45 a | 475.2 ± 16.5 a | 346.3 ± 14.9 a | 50.6 ± 1.76 a | 20.1 ± 1.28 abc | 451.1 ± 15.7 abc | 328.2 ± 14.5 abc | 46.2 ± 1.65 bcd |
| SNP | 21.3 ± 1.40 ab | 471.2 ± 16.4 ab | 340.3 ± 14.8 ab | 49.3 ± 1.69 ab | 19.7 ± 1.25 abc | 445.4 ± 15.6 abc | 320.4 ± 14.2 abc | 45.1 ± 1.51 bcde |
| NaHS | 20.9 ± 1.34 abc | 469.3 ± 16.0 ab | 339.6 ± 14.6 ab | 48.7 ± 1.60 abc | 19.1 ± 1.19 abc | 442.4 ± 15.1 abc | 319.7 ± 13.8 abc | 44.3 ± 1.40 cdef |
| HS + Eth | 19.5 ± 1.24 abc | 461.3 ± 15.9 abc | 315.6 ± 14.4 abc | 46.2 ± 1.55 bcd | 18.1 ± 1.13 bcd | 437.4 ± 14.9 bcd | 298.3 ± 13.7 bcd | 42.8 ± 1.37 def |
| HS + SNP | 19.2 ± 1.13 abc | 459.3 ± 15.9 abc | 312.9 ± 14.1 abc | 45.8 ± 1.45 bcd | 17.9 ± 1.10 bcde | 433.2 ± 14.8 bcd | 296.2 ± 13.6 bcd | 41.4 ± 1.35 ef |
| HS + NaHS | 18.8 ± 1.11 abc | 457.1 ± 15.4 abc | 311.9 ± 14.0 abc | 44.5 ± 1.40 cde | 17.4 ± 1.06 cdef | 431.6 ± 14.2 cd | 292.0 ± 12.4 cd | 40.2 ± 1.30 f |
| HS + Eth + HT | 13.1 ± 0.90 ghi | 311.6 ± 13.8 efgh | 230.8 ± 12.9 efgh | 29.4 ± 1.19 hij | 12.2 ± 0.85 ghij | 294.4 ± 12.9 efgh | 220.8 ± 11.3 efgh | 26.9 ± 1.13 ijk |
| HS + SNP + HT | 12.2 ± 0.83 ghij | 302.6 ± 13.7 efgh | 226.3 ± 12.5 efgh | 28.1 ± 1.15 hijk | 11.4 ± 0.77 hij | 289.1 ± 12.5 fgh | 214.3 ± 11.2 fgh | 25.4 ± 1.11 jkl |
| HS + NaHS + NBD | 14.6 ± 0.96 efgh | 361.7 ± 14.9 ef | 247.7 ± 13.7 ef | 31.9 ± 1.25 gh | 13.7 ± 0.91 gh | 341.2 ± 13.7 efgh | 233.8 ± 12.1 efgh | 29.1 ± 1.22 hij |
| HT + NaHS + cPTIO | 14.3 ± 0.92 fgh | 340.5 ± 14.7 efg | 242.7 ± 13.4 efg | 30.6 ± 1.20 ghi | 13.3 ± 0.88 gh | 323.1 ± 13.6 efgh | 227.8 ± 11.4 efgh | 28.2 ± 1.19 hijk |
| Treatments | Actual Efficiency of PSII | Maximum Efficiency of PSII | Intrinsic Efficiency of PSII | Photochemical Quenching | Non-Photochemical Quenching | Electron Transport Rate |
|---|---|---|---|---|---|---|
| Control | 0.621 ± 0.036 bcdef | 0.808 ± 0.051 bcdef | 0.731 ± 0.045 abcde | 0.684 ± 0.045 abcdefghi | 0.573 ± 0.032 cdefgh | 164.6 ± 9.4 e |
| HS | 0.497 ± 0.021 gh | 0.622 ± 0.029 gh | 0.620 ± 0.025 de | 0.569 ± 0.029 i | 0.778 ± 0.052 ab | 132.8 ± 6.8 fg |
| Eth | 0.751 ± 0.060 a | 0.876 ± 0.071 a | 0.849 ± 0.068 a | 0.831 ± 0.067 a | 0.455 ± 0.021 h | 241.3 ± 11.2 a |
| SNP | 0.744 ± 0.059 a | 0.869 ± 0.069 a | 0.841 ± 0.066 a | 0.824 ± 0.063 ab | 0.463 ± 0.025 gh | 237.6 ± 10.2 ab |
| NaHS | 0.732 ± 0.052 ab | 0.861 ± 0.065 ab | 0.837 ± 0.061 ab | 0.816 ± 0.060 abc | 0.469 ± 0.029 gh | 231.8 ± 10.1 abc |
| HS + Eth | 0.711 ± 0.048 abc | 0.849 ± 0.062 abc | 0.796 ± 0.058 abc | 0.796 ± 0.058 abcde | 0.582 ± 0.034 cdefgh | 220.7 ± 9.7 abcd |
| HS + SNP | 0.707 ± 0.045 abc | 0.836 ± 0.059 abc | 0.789 ± 0.051 abc | 0.787 ± 0.055 abcdef | 0.591 ± 0.036 cdefg | 216.5 ± 9.6 abcd |
| HS + NaHS | 0.701 ± 0.040 abc | 0.830 ± 0.053 abc | 0.781 ± 0.048 abcd | 0.773 ± 0.051 abcdefg | 0.598 ± 0.038 cdefg | 212.4 ± 9.5 abcd |
| HS + Eth + HT | 0.563 ± 0.028 efgh | 0.777 ± 0.039 efgh | 0.695 ± 0.035 abcde | 0.642 ± 0.036 efghi | 0.651 ± 0.046 bc | 147.6 ± 8.4 efg |
| HS + SNP + HT | 0.547 ± 0.024 fgh | 0.769 ± 0.034 fgh | 0.686 ± 0.033 abcde | 0.631 ± 0.033 fghi | 0.660 ± 0.048 bc | 141.9 ± 7.7 efg |
| HS + NaHS + NBD | 0.598 ± 0.033 cdefg | 0.795 ± 0.047 cdefg | 0.727 ± 0.042 abcde | 0.671 ± 0.043 bcdefghi | 0.623 ± 0.040 cd | 159.3 ± 8.8 ef |
| HS + NaHS + cPTIO | 0.582 ± 0.030 defgh | 0.786 ± 0.042 defgh | 0.721 ± 0.039 abcde | 0.664 ± 0.040 cdefghi | 0.639 ± 0.043 c | 152.4 ± 8.6 ef |
| Treatments | Actual Efficiency of PSII | Maximum Efficiency of PSII | Intrinsic Efficiency of PSII | Photochemical Quenching | Non-Photochemical Quenching | Electron Transport Rate |
|---|---|---|---|---|---|---|
| Control | 0.611 ± 0.025 bcdefg | 0.797 ± 0.042 bcdefg | 0.721 ± 0.038 abcde | 0.671 ± 0.037 bcdefghi | 0.582 ± 0.035 cdefgh | 153.7 ± 8.6 ef |
| HS | 0.472 ± 0.015 h | 0.601 ± 0.021 h | 0.598 ± 0.018 e | 0.543 ± 0.021 i | 0.799 ± 0.055 a | 121.4 ± 6.4 g |
| Eth | 0.725 ± 0.040 ab | 0.854 ± 0.067 ab | 0.826 ± 0.066 ab | 0.811 ± 0.056 abcd | 0.471 ± 0.027 fgh | 220.1 ± 10.7 abcd |
| SNP | 0.719 ± 0.036 ab | 0.847 ± 0.062 ab | 0.820 ± 0.063 ab | 0.801 ± 0.054 abcd | 0.483 ± 0.031 efgh | 215.8 ± 10.3 abcd |
| NaHS | 0.709 ± 0.034 abc | 0.839 ± 0.060 abc | 0.814 ± 0.059 abc | 0.798 ± 0.051 abcde | 0.492 ± 0.033 defgh | 210.6 ± 9.5 bcd |
| HS + Eth | 0.687 ± 0.032 abcd | 0.825 ± 0.058 abcd | 0.769 ± 0.051 abcd | 0.775 ± 0.048 abcdefg | 0.597 ± 0.037 cdefg | 202.5 ± 9.2 cd |
| HS + SNP | 0.679 ± 0.029 abcde | 0.819 ± 0.052 abcde | 0.758 ± 0.047 abcde | 0.769 ± 0.045 abcdefg | 0.606 ± 0.039 cdef | 198.3 ± 9.1 d |
| HS + NaHS | 0.674 ± 0.026 abcde | 0.811 ± 0.047 abcde | 0.749 ± 0.042 abcde | 0.754 ± 0.040 abcdefgh | 0.611 ± 0.041 cde | 191.4 ± 8.9 d |
| HS + Eth + HT | 0.538 ± 0.021 fgh | 0.760 ± 0.028 fgh | 0.676 ± 0.026 bcde | 0.624 ± 0.030 ghi | 0.667 ± 0.050 bc | 136.5 ± 8.1 efg |
| HS + SNP + HT | 0.529 ± 0.018 fgh | 0.751 ± 0.023 fgh | 0.654 ± 0.021 cde | 0.611 ± 0.027 hi | 0.679 ± 0.052 abc | 130.8 ± 7.4 fg |
| HS + NaHS + NBD | 0.576 ± 0.023 defgh | 0.780 ± 0.038 defgh | 0.705 ± 0.033 abcde | 0.656 ± 0.034 defghi | 0.635 ± 0.044 c | 146.6 ± 8.4 efg |
| HS + NaHS + cPTIO | 0.563 ± 0.022 efgh | 0.771 ± 0.030 efgh | 0.694 ± 0.030 abcde | 0.642 ± 0.032 efghi | 0.642 ± 0.046 c | 141.2 ± 8.2 efg |
| Treatments | H2O2 Content | TBARS Content | SOD Activity | APX Activity | GR Activity |
|---|---|---|---|---|---|
| (nmol g−1 FW) | (U mg−1 Protein min−1) | ||||
| Control | 28.3 ± 1.26 ghi | 10.6 ± 1.22 efghijk | 7.41 ± 0.43 mn | 1.35 ± 0.10 mn | 0.19 ± 0.01 jkl |
| HS | 86.7 ± 4.14 b | 22.1 ± 2.07 a | 11.0 ± 0.55 fghij | 1.94 ± 0.14 jklm | 0.27 ± 0.017 ghi |
| Eth | 21.1 ± 1.17 i | 7.1 ± 0.79 k | 13.9 ± 0.76 bcd | 2.97 ± 0.23 bcdef | 0.38 ± 0.028 bcd |
| SNP | 23.5 ± 1.20 hi | 7.9 ± 0.81 jk | 13.1 ± 0.62 cde | 2.73 ± 0.22 cdefgh | 0.36 ± 0.026 bcd |
| NaHS | 24.7 ± 1.22 ghi | 8.2 ± 0.97 ijk | 12.8 ± 0.50 cdef | 2.59 ± 0.20 efghi | 0.34 ± 0.025 cdef |
| HS + Eth | 29.4 ± 1.72 ghi | 10.9 ± 1.20 efghijk | 15.8 ± 0.98 a | 3.74 ± 0.29 a | 0.46 ± 0.034 a |
| HS + SNP | 30.2 ± 2.11 gh | 11.2 ± 1.25 efghijk | 14.9 ± 0.81 ab | 3.53 ± 0.26 ab | 0.42 ± 0.032 ab |
| HS + NaHS | 30.9 ± 2.51 gh | 11.5 ± 1.34 defghijk | 14.1 ± 0.79 bc | 3.32 ± 0.24 abc | 0.40 ± 0.030 abc |
| HS + Eth + HT | 61.3 ± 3.16 cde | 14.2 ± 1.55 bcdefg | 10.8 ± 0.49 ghij | 1.91 ± 0.16 jklm | 0.25 ± 0.015 hij |
| HS + SNP + HT | 64.1 ± 3.37 cd | 14.7 ± 1.65 bcdef | 9.7 ± 0.40 jkl | 1.87 ± 0.13 jklm | 0.24 ± 0.011 hijk |
| HS + NaHS + NBD | 51.8 ± 2.70 f | 13.3 ± 1.36 bcdefgh | 12.3 ± 0.65 cdefgh | 2.31 ± 0.19 ghijk | 0.29 ± 0.023 efgh |
| HS + NaHS + cPTIO | 53.2 ± 2.80 ef | 13.9 ± 1.49 bcdefg | 12.1 ± 0.58 defgh | 2.19 ± 0.18 hijkl | 0.28 ± 0.020 fgh |
| Treatments | H2O2 Content | TBARS Content | SOD Activity | APX Activity | GR Activity |
|---|---|---|---|---|---|
| (nmol g−1 FW) | (U mg−1 Protein min−1) | ||||
| Control | 30.5 ± 1.85 gh | 12.2 ± 1.13 cdefghij | 6.38 ± 0.21 n | 1.24 ± 0.07 n | 0.16 ± 0.005 l |
| HS | 97.9 ± 5.46 a | 26.1 ± 2.11 a | 9.31 ± 0.34 jkl | 1.76 ± 0.12 klmn | 0.21 ± 0.011 ijkl |
| Eth | 24.6 ± 1.19 ghi | 8.5 ± 0.73 hijk | 11.7 ± 0.55 efghi | 2.67 ± 0.19 defghi | 0.29 ± 0.017 efgh |
| SNP | 26.9 ± 1.24 ghi | 9.7 ± 0.87 ghijk | 10.9 ± 0.51 ghij | 2.44 ± 0.17 fghij | 0.28 ± 0.015 fgh |
| NaHS | 28.0 ± 1.32 ghi | 10.0 ± 1.02 fghijk | 10.5 ± 0.45 hijk | 2.32 ± 0.16 ghijk | 0.26 ± 0.011 hi |
| HS + Eth | 31.8 ± 2.46 gh | 12.8 ± 1.22 bcdefghi | 13.2 ± 0.66 bcde | 3.27 ± 0.24 abcd | 0.38± 0.026 bcd |
| HS + SNP | 32.9 ± 2.60 g | 13.3 ± 1.30 bcdefgh | 12.6 ± 0.61 cdefg | 3.10 ± 0.21 bcde | 0.35 ± 0.023 cde |
| HS + NaHS | 33.2 ± 2.88 g | 13.8 ± 1.53 bcdefg | 11.9 ± 0.57 efghi | 2.90 ± 0.20 cdefg | 0.33 ± 0.020 defg |
| HS + Eth + HT | 65.8 ± 3.56 cd | 16.9 ± 1.92 bc | 8.8 ± 0.30 klm | 1.71 ± 0.13 klmn | 0.19 ± 0.01 jkl |
| HS + SNP + HT | 69.7 ± 4.05 c | 17.3 ± 2.04 b | 8.0 ± 0.25 lmn | 1.67 ± 0.11 lmn | 0.18 ± 0.01 kl |
| HS + NaHS + NBD | 57.4 ± 2.90 def | 15.4 ± 1.72 bcde | 10.1 ± 0.40 ijk | 2.05 ± 0.18 ijkl | 0.24 ± 0.015 hijk |
| HS + NaHS + cPTIO | 59.3 ± 3.17 def | 16.2 ± 1.81 bcd | 9.7 ± 0.35 jkl | 1.96 ± 0.16 jklm | 0.23 ± 0.011 hijk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, H.; Fatma, M.; Sehar, Z.; Mir, I.R.; Khan, N.A. Hydrogen Sulfide, Ethylene, and Nitric Oxide Regulate Redox Homeostasis and Protect Photosynthetic Metabolism under High Temperature Stress in Rice Plants. Antioxidants 2022, 11, 1478. https://doi.org/10.3390/antiox11081478
Gautam H, Fatma M, Sehar Z, Mir IR, Khan NA. Hydrogen Sulfide, Ethylene, and Nitric Oxide Regulate Redox Homeostasis and Protect Photosynthetic Metabolism under High Temperature Stress in Rice Plants. Antioxidants. 2022; 11(8):1478. https://doi.org/10.3390/antiox11081478
Chicago/Turabian StyleGautam, Harsha, Mehar Fatma, Zebus Sehar, Iqbal R. Mir, and Nafees A. Khan. 2022. "Hydrogen Sulfide, Ethylene, and Nitric Oxide Regulate Redox Homeostasis and Protect Photosynthetic Metabolism under High Temperature Stress in Rice Plants" Antioxidants 11, no. 8: 1478. https://doi.org/10.3390/antiox11081478