Measurement of Tetrahydrobiopterin in Animal Tissue Samples by HPLC with Electrochemical Detection—Protocol Optimization and Pitfalls
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Stability of BH4 and BH2 Standard Stock Solutions under Different Storage Conditions
2.3. Animals
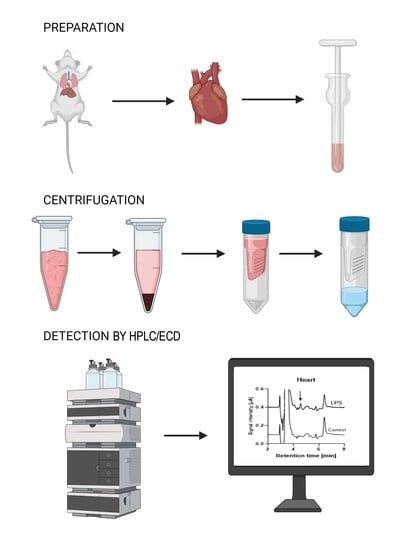
2.4. HPLC/ECD
2.5. Animal Tissue Preparation
2.6. Statistical Analysis
3. Results
3.1. Standard Curve
3.2. Stability of Standards during Storage
3.3. Oxidation of Standards
3.4. Animal Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kappock, T.J.; Caradonna, J.P. Pterin-dependent amino acid hydroxylases. Chem. Rev. 1996, 96, 2659–2756. [Google Scholar] [CrossRef]
- Vasquez-Vivar, J.; Kalyanaraman, B.; Martasek, P.; Hogg, N.; Masters, B.S.; Karoui, H.; Tordo, P.; Pritchard, K.A., Jr. Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc. Natl. Acad. Sci. USA 1998, 95, 9220–9225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaub, J.; Daumling, S.; Curtius, H.C.; Niederwieser, A.; Bartholome, K.; Viscontini, M.; Schircks, B.; Bieri, J.H. Tetrahydrobiopterin therapy of atypical phenylketonuria due to defective dihydrobiopterin biosynthesis. Arch. Dis. Child. 1978, 53, 674–676. [Google Scholar] [CrossRef] [Green Version]
- Bendall, J.K.; Douglas, G.; McNeill, E.; Channon, K.M.; Crabtree, M.J. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid. Redox Signal. 2014, 20, 3040–3077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiber, A.; Oelze, M.; Daub, S.; Steven, S.; Schuff, A.; Kroller-Schon, S.; Hausding, M.; Wenzel, P.; Schulz, E.; Gori, T.; et al. Vascular redox signaling, redox switches in endothelial nitric oxide synthase and endothelial dysfunction. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1177–1211. [Google Scholar]
- Schulz, E.; Jansen, T.; Wenzel, P.; Daiber, A.; Munzel, T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid. Redox Signal. 2008, 10, 1115–1126. [Google Scholar] [CrossRef]
- Harrison, D.G.; Chen, W.; Dikalov, S.; Li, L. Regulation of endothelial cell tetrahydrobiopterin pathophysiological and therapeutic implications. Adv. Pharmacol. 2010, 60, 107–132. [Google Scholar]
- Vasquez-Vivar, J. Tetrahydrobiopterin, superoxide, and vascular dysfunction. Free Radic. Biol. Med. 2009, 47, 1108–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landmesser, U.; Dikalov, S.; Price, S.R.; McCann, L.; Fukai, T.; Holland, S.M.; Mitch, W.E.; Harrison, D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Investig. 2003, 111, 1201–1209. [Google Scholar] [CrossRef]
- Guzik, T.J.; Mussa, S.; Gastaldi, D.; Sadowski, J.; Ratnatunga, C.; Pillai, R.; Channon, K.M. Mechanisms of increased vascular superoxide production in human diabetes mellitus: Role of nad(p)h oxidase and endothelial nitric oxide synthase. Circulation 2002, 105, 1656–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alp, N.J.; Mussa, S.; Khoo, J.; Cai, S.; Guzik, T.; Jefferson, A.; Goh, N.; Rockett, K.A.; Channon, K.M. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic gtp-cyclohydrolase i overexpression. J. Clin. Investig. 2003, 112, 725–735. [Google Scholar] [CrossRef]
- Bendall, J.K.; Alp, N.J.; Warrick, N.; Cai, S.; Adlam, D.; Rockett, K.; Yokoyama, M.; Kawashima, S.; Channon, K.M. Stoichiometric relationships between endothelial tetrahydrobiopterin, endothelial no synthase (enos) activity, and enos coupling in vivo: Insights from transgenic mice with endothelial-targeted gtp cyclohydrolase 1 and enos overexpression. Circ. Res. 2005, 97, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Laursen, J.B.; Somers, M.; Kurz, S.; McCann, L.; Warnholtz, A.; Freeman, B.A.; Tarpey, M.; Fukai, T.; Harrison, D.G. Endothelial regulation of vasomotion in apoe-deficient mice: Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 2001, 103, 1282–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003, 278, 22546–22554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alp, N.J.; Channon, K.M. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 413–420. [Google Scholar] [CrossRef]
- Munzel, T.; Daiber, A. Does endothelial tetrahydrobiopterin control the endothelial no synthase coupling state in arterial resistance arteries? Br. J. Pharmacol. 2017, 174, 2422–2424. [Google Scholar] [CrossRef] [Green Version]
- Chuaiphichai, S.; Crabtree, M.J.; McNeill, E.; Hale, A.B.; Trelfa, L.; Channon, K.M.; Douglas, G. A key role for tetrahydrobiopterin-dependent endothelial nos regulation in resistance arteries: Studies in endothelial cell tetrahydrobiopterin-deficient mice. Br. J. Pharmacol. 2017, 174, 657–671. [Google Scholar] [CrossRef] [Green Version]
- Kossmann, S.; Hu, H.; Steven, S.; Schonfelder, T.; Fraccarollo, D.; Mikhed, Y.; Brahler, M.; Knorr, M.; Brandt, M.; Karbach, S.H.; et al. Inflammatory monocytes determine endothelial nitric-oxide synthase uncoupling and nitro-oxidative stress induced by angiotensin ii. J. Biol. Chem. 2014, 289, 27540–27550. [Google Scholar] [CrossRef] [Green Version]
- Hashiguchi, T.; Kakihana, Y.; Isowaki, S.; Kuniyoshi, T.; Kaminosono, T.; Nagata, E.; Tobo, K.; Tahara, M.; Okayama, N.; Arakawa, Y.; et al. Systematic evaluation of nitric oxide, tetrahydrobiopterin, and anandamide levels in a porcine model of endotoxemia. J. Anesth. 2008, 22, 213–220. [Google Scholar] [CrossRef]
- Galley, H.F.; Le Cras, A.E.; Yassen, K.; Grant, I.S.; Webster, N.R. Circulating tetrahydrobiopterin concentrations in patients with septic shock. Br. J. Anaesth. 2001, 86, 578–580. [Google Scholar] [CrossRef] [Green Version]
- Hattori, Y.; Nakanishi, N.; Kasai, K.; Murakami, Y.; Shimoda, S. Tetrahydrobiopterin and gtp cyclohydrolase i in a rat model of endotoxic shock: Relation to nitric oxide synthesis. Exp. Physiol. 1996, 81, 665–671. [Google Scholar] [CrossRef]
- Starr, A.; Sand, C.A.; Heikal, L.; Kelly, P.D.; Spina, D.; Crabtree, M.; Channon, K.M.; Leiper, J.M.; Nandi, M. Overexpression of gtp cyclohydrolase 1 feedback regulatory protein is protective in a murine model of septic shock. Shock 2014, 42, 432–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guibal, P.; Lo, A.; Maitre, P.; Moussa, F. Pterin determination in cerebrospinal fluid: State of the art. Pteridines 2017, 28, 83–89. [Google Scholar] [CrossRef]
- Oelze, M.; Knorr, M.; Schuhmacher, S.; Heeren, T.; Otto, C.; Schulz, E.; Reifenberg, K.; Wenzel, P.; Munzel, T.; Daiber, A. Vascular dysfunction in streptozotocin-induced experimental diabetes strictly depends on insulin deficiency. J. Vasc. Res. 2011, 48, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Oelze, M.; Kroller-Schon, S.; Welschof, P.; Jansen, T.; Hausding, M.; Mikhed, Y.; Stamm, P.; Mader, M.; Zinssius, E.; Agdauletova, S.; et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS ONE 2014, 9, e112394. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Hausding, M.; Kroller-Schon, S.; Mader, M.; Mikhed, Y.; Stamm, P.; Zinssius, E.; Pfeffer, A.; Welschof, P.; Agdauletova, S.; et al. Gliptin and glp-1 analog treatment improves survival and vascular inflammation/dysfunction in animals with lipopolysaccharide-induced endotoxemia. Basic Res. Cardiol. 2015, 110, 6. [Google Scholar] [CrossRef]
- Kroller-Schon, S.; Knorr, M.; Hausding, M.; Oelze, M.; Schuff, A.; Schell, R.; Sudowe, S.; Scholz, A.; Daub, S.; Karbach, S.; et al. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc. Res. 2012, 96, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Vladic, N.; Ge, Z.D.; Leucker, T.; Brzezinska, A.K.; Du, J.H.; Shi, Y.; Warltier, D.C.; Pratt, P.F., Jr.; Kersten, J.R. Decreased tetrahydrobiopterin and disrupted association of hsp90 with enos by hyperglycemia impair myocardial ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2130–H2139. [Google Scholar] [CrossRef] [Green Version]
- Amour, J.; Brzezinska, A.K.; Jager, Z.; Sullivan, C.; Weihrauch, D.; Du, J.; Vladic, N.; Shi, Y.; Warltier, D.C.; Pratt, P.F., Jr.; et al. Hyperglycemia adversely modulates endothelial nitric oxide synthase during anesthetic preconditioning through tetrahydrobiopterin- and heat shock protein 90-mediated mechanisms. Anesthesiology 2010, 112, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Chander, P.N.; Gealekman, O.; Brodsky, S.V.; Elitok, S.; Tojo, A.; Crabtree, M.; Gross, S.S.; Goligorsky, M.S. Nephropathy in zucker diabetic fat rat is associated with oxidative and nitrosative stress: Prevention by chronic therapy with a peroxynitrite scavenger ebselen. J. Am. Soc. Nephrol. 2004, 15, 2391–2403. [Google Scholar] [CrossRef] [Green Version]
- Bailey, J.D.; Shaw, A.; McNeill, E.; Nicol, T.; Diotallev, M.; Chuaiphichai, S.; Patel, J.; Hale, A.; Channon, K.M.; Crabtree, M.J. Isolation and culture of murine bone marrow-derived macrophages for nitric oxide and redox biology. Nitric Oxide 2020, 100–101, 17–29. [Google Scholar] [CrossRef]
- Whitsett, J.; Picklo, M.J., Sr.; Vasquez-Vivar, J. 4-hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated gtp cyclohydrolase proteasomal degradation. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2340–2347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosentino, F.; Barker, J.E.; Brand, M.P.; Heales, S.J.; Werner, E.R.; Tippins, J.R.; West, N.; Channon, K.M.; Volpe, M.; Luscher, T.F. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 496–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, Y.S.; Mori, K.; Nakashima, A.; Nagatsu, I.; Nagatsu, T.; Ota, A. Determination of tetrahydrobiopterin in murine locus coeruleus by hplc with fluorescence detection. Brain Res. Protoc. 2001, 8, 25–31. [Google Scholar] [CrossRef]
- Blackwell, K.A.; Sorenson, J.P.; Richardson, D.M.; Smith, L.A.; Suda, O.; Nath, K.; Katusic, Z.S. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: Role of tetrahydrobiopterin. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2448–H2453. [Google Scholar] [CrossRef] [Green Version]
- d’Uscio, L.V.; Katusic, Z.S. Increased vascular biosynthesis of tetrahydrobiopterin in apolipoprotein e-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2466–H2471. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.J.; Lin, L.; Zeng, J.S.; Huang, R.X.; Channon, K.M.; Chen, A.F. Endothelium-targeted transgenic gtp-cyclohydrolase i overexpression inhibits neointima formation in mouse carotid artery. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1260–1266. [Google Scholar] [CrossRef]
- Crabtree, M.J.; Smith, C.L.; Lam, G.; Goligorsky, M.S.; Gross, S.S. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in no vs. Superoxide production by enos. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1530–H1540. [Google Scholar] [CrossRef] [Green Version]
- Azizi, M.Y.; Chik, Z. Bioanalysis of tetrahydrobiopterin with liquid chromatographic-mass spectrometric and its application for pharmacokinetics in apolipoprotein e knockout mice. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 494–501. [Google Scholar] [CrossRef]
- Yuan, T.F.; Huang, H.Q.; Gao, L.; Wang, S.T.; Li, Y. A novel and reliable method for tetrahydrobiopterin quantification: Benzoyl chloride derivatization coupled with liquid chromatography-tandem mass spectrometry analysis. Free Radic. Biol. Med. 2018, 118, 119–125. [Google Scholar] [CrossRef]
- Wainwright, M.S.; Arteaga, E.; Fink, R.; Ravi, K.; Chace, D.H.; Black, S.M. Tetrahydrobiopterin and nitric oxide synthase dimer levels are not changed following hypoxia-ischemia in the newborn rat. Dev. Brain Res. 2005, 156, 183–192. [Google Scholar] [CrossRef]
- Deng, C.; Wang, S.; Niu, Z.; Ye, Y.; Gao, L. Newly established lc-ms/ms method for measurement of plasma bh4 as a predictive biomarker for kidney injury in diabetes. Free Radic. Biol. Med. 2022, 178, 1–6. [Google Scholar] [CrossRef]
- Fukushima, T.; Nixon, J.C. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal. Biochem. 1980, 102, 176–188. [Google Scholar] [CrossRef]
- Blau, N.; Kierat, L.; Heizmann, C.W.; Endres, W.; Giudici, T.; Wang, M. Screening for tetrahydrobiopterin deficiency in newborns using dried urine on filter paper. J. Inherit. Metab. Dis. 1992, 15, 402–404. [Google Scholar] [CrossRef]
- Ormazabal, A.; Garcia-Cazorla, A.; Fernandez, Y.; Fernandez-Alvarez, E.; Campistol, J.; Artuch, R. Hplc with electrochemical and fluorescence detection procedures for the diagnosis of inborn errors of biogenic amines and pterins. J. Neurosci. Methods 2005, 142, 153–158. [Google Scholar] [CrossRef]
- Hyland, K. Estimation of tetrahydro, dihydro and fully oxidised pterins by high-performance liquid chromatography using sequential electrochemical and fluorometric detection. J. Chromatogr. 1985, 343, 35–41. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Mortensen, A.; Hasselholt, S.; Tveden-Nyborg, P.; Lykkesfeldt, J. Guinea pig ascorbate status predicts tetrahydrobiopterin plasma concentration and oxidation ratio in vivo. Nutr. Res. 2013, 33, 859–867. [Google Scholar] [CrossRef] [Green Version]
- Capeillere-Blandin, C.; Mathieu, D.; Mansuy, D. Reduction of ferric haemoproteins by tetrahydropterins: A kinetic study. Biochem. J. 2005, 392, 583–587. [Google Scholar] [CrossRef] [Green Version]
- Whitsett, J.; Rangel Filho, A.; Sethumadhavan, S.; Celinska, J.; Widlansky, M.; Vasquez-Vivar, J. Human endothelial dihydrofolate reductase low activity limits vascular tetrahydrobiopterin recycling. Free Radic. Biol. Med. 2013, 63, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Fisher, A.E.; Maxwell, S.C.; Naughton, D.P. Superoxide and hydrogen peroxide suppression by metal ions and their edta complexes. Biochem. Biophys. Res. Commun. 2004, 316, 48–51. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Q.; Melendez, V.; Weina, P. Comparison of hplc with electrochemical detection and lc-ms/ms for the separation and validation of artesunate and dihydroartemisinin in animal and human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 867, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Arning, E.; Bottiglieri, T. Lc-ms/ms analysis of cerebrospinal fluid metabolites in the pterin biosynthetic pathway. JIMD Rep. 2016, 29, 1–9. [Google Scholar] [PubMed] [Green Version]
- Lunte, C.E.; Kissinger, P.T. Determination of pterins in biological samples by liquid chromatography/electrochemistry with a dual-electrode detector. Anal. Chem. 1983, 55, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Lunte, C.E.; Kissinger, P.T. The determination of pterins in biological samples by liquid chromatography/electrochemistry. Anal. Biochem. 1983, 129, 377–386. [Google Scholar] [CrossRef]
- Biondi, R.; Ambrosio, G.; De Pascali, F.; Tritto, I.; Capodicasa, E.; Druhan, L.J.; Hemann, C.; Zweier, J.L. Hplc analysis of tetrahydrobiopterin and its pteridine derivatives using sequential electrochemical and fluorimetric detection: Application to tetrahydrobiopterin autoxidation and chemical oxidation. Arch. Biochem. Biophys. 2012, 520, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Fismen, L.; Eide, T.; Djurhuus, R.; Svardal, A.M. Simultaneous quantification of tetrahydrobiopterin, dihydrobiopterin, and biopterin by liquid chromatography coupled electrospray tandem mass spectrometry. Anal. Biochem. 2012, 430, 163–170. [Google Scholar] [CrossRef]
- Khan, A.; Bhojani, M.; Rajput, M.; Ponnuri, R.; Biswas, A.; Khan, A. Development and validation of a liquid chromatography-tandem mass spectrometry method for direct determination of tetrahydrobiopterin—An enzyme cofactor in human plasma. Int. J. Pharm. Chem. Sci. 2013, 2, 695–704. [Google Scholar]
- Tomsikova, H.; Tomsik, P.; Solich, P.; Novakova, L. Determination of pteridines in biological samples with an emphasis on their stability. Bioanalysis 2013, 5, 2307–2326. [Google Scholar] [CrossRef]
- Werner, E.R.; Blau, N.; Thony, B. Tetrahydrobiopterin: Biochemistry and pathophysiology. Biochem. J. 2011, 438, 397–414. [Google Scholar] [CrossRef] [Green Version]
- Chalupsky, K.; Cai, H. Endothelial dihydrofolate reductase: Critical for nitric oxide bioavailability and role in angiotensin ii uncoupling of endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2005, 102, 9056–9061. [Google Scholar] [CrossRef] [Green Version]
- Ionova, I.A.; Vasquez-Vivar, J.; Whitsett, J.; Herrnreiter, A.; Medhora, M.; Cooley, B.C.; Pieper, G.M. Deficient bh4 production via de novo and salvage pathways regulates no responses to cytokines in adult cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2178–H2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munzel, T.; Daiber, A. Redox regulation of dihydrofolate reductase: Friend or troublemaker? Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2261–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kroller-Schon, S.; Munzel, T.; Li, H. New therapeutic implications of endothelial nitric oxide synthase (enos) function/dysfunction in cardiovascular disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.H.; Guan, Y.Y.; Alp, N.J.; Channon, K.M.; Chen, A.F. Endothelium-specific gtp cyclohydrolase i overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation 2008, 117, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Gori, T.; Bruno, R.M.; Taddei, S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart J. 2010, 31, 2741–2748. [Google Scholar] [CrossRef] [Green Version]
- Vasquez-Vivar, J.; Shi, Z.; Luo, K.; Thirugnanam, K.; Tan, S. Tetrahydrobiopterin in antenatal brain hypoxia-ischemia-induced motor impairments and cerebral palsy. Redox Biol. 2017, 13, 594–599. [Google Scholar] [CrossRef]
- Vasquez-Vivar, J.; Shi, Z.; Jeong, J.W.; Luo, K.; Sharma, A.; Thirugnanam, K.; Tan, S. Neuronal vulnerability to fetal hypoxia-reoxygenation injury and motor deficit development relies on regional brain tetrahydrobiopterin levels. Redox Biol. 2020, 29, 101407. [Google Scholar] [CrossRef]
- Liu, Y.; Baumgardt, S.L.; Fang, J.; Shi, Y.; Qiao, S.; Bosnjak, Z.J.; Vasquez-Vivar, J.; Xia, Z.; Warltier, D.C.; Kersten, J.R.; et al. Transgenic overexpression of gtp cyclohydrolase 1 in cardiomyocytes ameliorates post-infarction cardiac remodeling. Sci. Rep. 2017, 7, 3093. [Google Scholar] [CrossRef] [Green Version]
- Sethumadhavan, S.; Whitsett, J.; Bennett, B.; Ionova, I.A.; Pieper, G.M.; Vasquez-Vivar, J. Increasing tetrahydrobiopterin in cardiomyocytes adversely affects cardiac redox state and mitochondrial function independently of changes in no production. Free Radic. Biol. Med. 2016, 93, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Maglione, M.; Cardini, B.; Oberhuber, R.; Watschinger, K.; Jenny, M.; Gostner, J.; Hermann, M.; Obrist, P.; Margreiter, R.; Pratschke, J.; et al. Prevention of lethal murine pancreas ischemia reperfusion injury is specific for tetrahydrobiopterin. Transpl. Int. 2012, 25, 1084–1095. [Google Scholar] [CrossRef] [Green Version]
- Oelze, M.; Mollnau, H.; Hoffmann, N.; Warnholtz, A.; Bodenschatz, M.; Smolenski, A.; Walter, U.; Skatchkov, M.; Meinertz, T.; Munzel, T. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cgmp signaling and endothelial dysfunction. Circ. Res. 2000, 87, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radi, R.; Peluffo, G.; Alvarez, M.N.; Naviliat, M.; Cayota, A. Unraveling peroxynitrite formation in biological systems. Free Radic. Biol. Med. 2001, 30, 463–488. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.; Wu, Y.; Song, P.; Zou, M.H. Tyrosine nitration of pa700 activates the 26s proteasome to induce endothelial dysfunction in mice with angiotensin ii-induced hypertension. Hypertension 2009, 54, 625–632. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Y.; Song, P.; Zhang, M.; Wang, S.; Zou, M.H. Proteasome-dependent degradation of guanosine 5’-triphosphate cyclohydrolase i causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation 2007, 116, 944–953. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Lu, Q.; Ding, Y.; Wang, Q.; Xiao, L.; Song, P.; Zou, M.H. Endothelial nitric oxide synthase-derived nitric oxide prevents dihydrofolate reductase degradation via promoting s-nitrosylation. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2366–2373. [Google Scholar] [CrossRef] [Green Version]
- Heitzer, T.; Brockhoff, C.; Mayer, B.; Warnholtz, A.; Mollnau, H.; Henne, S.; Meinertz, T.; Munzel, T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: Evidence for a dysfunctional nitric oxide synthase. Circ. Res. 2000, 86, E36–E41. [Google Scholar] [CrossRef] [Green Version]
- Heitzer, T.; Krohn, K.; Albers, S.; Meinertz, T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with type ii diabetes mellitus. Diabetologia 2000, 43, 1435–1438. [Google Scholar] [CrossRef] [Green Version]
- Antoniades, C.; Shirodaria, C.; Warrick, N.; Cai, S.; de Bono, J.; Lee, J.; Leeson, P.; Neubauer, S.; Ratnatunga, C.; Pillai, R.; et al. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: Effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation 2006, 114, 1193–1201. [Google Scholar] [CrossRef]
- Gori, T.; Burstein, J.M.; Ahmed, S.; Miner, S.E.; Al-Hesayen, A.; Kelly, S.; Parker, J.D. Folic acid prevents nitroglycerin-induced nitric oxide synthase dysfunction and nitrate tolerance: A human in vivo study. Circulation 2001, 104, 1119–1123. [Google Scholar] [CrossRef] [Green Version]
- Schuhmacher, S.; Wenzel, P.; Schulz, E.; Oelze, M.; Mang, C.; Kamuf, J.; Gori, T.; Jansen, T.; Knorr, M.; Karbach, S.; et al. Pentaerythritol tetranitrate improves angiotensin ii-induced vascular dysfunction via induction of heme oxygenase-1. Hypertension 2010, 55, 897–904. [Google Scholar] [CrossRef] [Green Version]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Munzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef]







| Parameters | Specification |
|---|---|
| HPLC system | Dionex UltiMate 3000 (Thermo Fisher Scientific) |
| Column | Synergi Polar, 250 mm × 4.6 mm, 4 µm, 80 Å (Phenomenex) |
| Column temperature | 37 °C |
| Mobile phase | a 50 mM potassium phosphate, pH 2.6, 0.1 mM DTE, 0.1 mM DTPA b 50 mM potassium phosphate, pH 4.5 |
| Flow rate | a 0.7 mL/min b 1.0 mL/min |
| Run time per sample | 15 min |
| Injection volume | 20 µL |
| Autosampler temperature | 4 °C |
| Detector | Dionex CoulArray™(Thermo Fisher Scientific) |
| Autosampler temperature | 4 °C |
| Detector settings | |
| - Electrode potentials (mV vs. palladium reference electrode) | a 0; +150; +280; +365; +600 b 0; +150; +280 |
| - BH4 quantification channel(s) | a 150 mV + 280 mV b 0 mV |
| - BH2 quantification channel(s) | a 600 mV b 280 mV |
| - Accuracy (deviation from the expected calibration curve value) b | 7.99% (0 mV, 1 µM BH4, n = 16) −3.11% (280 mV, 1 µM BH2, n = 15) |
| - Precision (deviation from the measured mean) b | 11.27% (0 mV, 1 µM BH4, n = 16) 3.36% (280 mV, 1 µM BH2, n = 15) |
| - Noise b | 3.13 ± 0.0.82 nA (0 mV, BH4, n = 10) 0.49 ± 0.0.16 nA (280 mV, BH2, n = 10) |
| - Limit of quantification, peak height and quantity of material (S/N = 10) b | 31.3 nA, 4.49 pmol (0 mV, BH4) 4.9 nA, 0.38 pmol (280 mV, BH2) |
| - Limit of detection, peak height and quantity of material (S/N = 3) b | 9.4 nA, 1.35 pmol (0 mV, BH4) 1.5 nA, 0.11 pmol (280 mV, BH2) |
| Species/Strain | BH4 [pmol/mg Protein] | BH2 [pmol/mg Protein] | Reference & Method | |||
|---|---|---|---|---|---|---|
| Wistar rats, healthy control and LPS-induced sepsis | Heart: Ctr 1: 0.3 LPS 2: 7.5 | Kidney: Ctr: 0.5 LPS: 5.2 | Brain: Ctr: 78 LPS: 133 | n.d. 3 | Present work HPLC/ECD | |
| Healthy C57BL/CBA mice, GTPCH-I-deficient 4 Hph-1 5 mice | Aorta: Ctr: 100–120 Hph-1: 50–60 | n.d. | [33] HPLC/ECD | |||
| Healthy C3/HeN mice and septic LPS-treated mice | Brain: Ctr: 4.2 LPS: 7 | n.d. | [34] HPLC/fluorescence with differential oxidation | |||
| Healthy C57BL/6 mice—young versus old | Aorta: Young: 8 Old: 5.8 | Aorta: Young: 6 Old: 4 | [35] HPLC/fluorescence with differential oxidation | |||
| C57BL/6 mice and ApoETm1Unc mice with Western diet (HFD 6) | Aorta: Ctr + HFD: 7 ApoE + HFD: 7–37 | Brain: Ctr + HFD: 20–25 ApoE + HFD: 25–30 | Endothelial cells Ctr + HFD: 10 ApoE + HFD: 25 | Aorta: Ctr + HFD: 5 ApoE + HFD: 5 | Brain: Ctr + HFD: 1–2 ApoE + HFD: 1–2 | [36] HPLC/fluorescence with differential oxidation |
| C57BL/6 mice and GTPCH-I overexpressing mice (tg-GCH 7) | Aorta: Ctr: 2.7 tg-GCH: 12 | Aorta: Ctr: 3.7 tg-GCH: 16 | [37] HPLC/fluorescence with differential oxidation | |||
| New Zealand white rabbits (healthy, hyperglycemic, treatments) | Heart: Ctr: 7.6; HG 8: 6; IPC 9: 10.2; HG + IPC: 7 Ctr + SEP 10: 11; HG + SEP: 6; IPC + SEP: 14; HG + IPC + SEP: 13 Ctr + DAHP 11: 6; IPC + DAHP: 10 | n.d. | [28] HPLC/ECD | |||
| Rats, healthy (ZL 12) versus diabetic (ZDF 13) | Kidney: ZL: 6.5 (22 w) ZDF: 2.5 (22 w) ZDF + ebselen: 6.5 (22 w) | Kidney: ZL: <1 (22 w) ZDF: 5 (22 w) ZDF + ebselen: <1 (22 w) | [30] HPLC/ECD | |||
| Rats, healthy (ZL) versus diabetic (ZDF) | Lung: ZL: 2.2 (8 w); ZL: 1.9 (22 w) ZDF: 1.9 (8 w); ZDF: 0.8 (22 w) ZDF + ebselen: 1.3 (22 w) | Lung: ZL: 0.1 (8 w); ZL: 0.3 (22 w) ZDF: 0.2 (8 w); ZDF: 1.1 (22 w) ZDF + ebselen: 0.4 (22 w) | [38] HPLC/ECD | |||
| C57BL/6 mice and DOCA 14 salt hypertension, treatments | Aorta: Ctr: 110; Ctr + BH4: 130 DOCA: 50; DOCA (p47phox−/−): 90; DOCA (eNOS−/−): 70; DOCA + BH4: 90 | n.d. | [9] HPLC/fluorescence with differential oxidation | |||
| ApoE−/− mice with oral versus i.v. BH4 administration | Plasma: Peak value (p.o.): 423 nmol/L Peak value (i.v.): 2004 nmol/L | n.d. | [39] LC/MS 15 | |||
| Healthy volunteers | Plasma: Age of 20: 19.5 nmol/L Age of 60: 6.6 nmol/L | n.d. | [40] LC/MS, derivatization with benzoyl chloride | |||
| Rats, healthy versus ischemia by ligation of the carotid artery | Brain: Sham: 4.5–7 Ischemia: 5–5.7 | n.d. | [41] LC/MS | |||
| Diabetic patients with kidney disease | Plasma: Normoalbuminuria: 21.6 nmol/L Microalbuminuria: 12.9 nmol/L Microalbuminuria: 5.0 nmol/L | n.d. | [42] LC/MS | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vujacic-Mirski, K.; Oelze, M.; Kuntic, I.; Kuntic, M.; Kalinovic, S.; Li, H.; Zielonka, J.; Münzel, T.; Daiber, A. Measurement of Tetrahydrobiopterin in Animal Tissue Samples by HPLC with Electrochemical Detection—Protocol Optimization and Pitfalls. Antioxidants 2022, 11, 1182. https://doi.org/10.3390/antiox11061182
Vujacic-Mirski K, Oelze M, Kuntic I, Kuntic M, Kalinovic S, Li H, Zielonka J, Münzel T, Daiber A. Measurement of Tetrahydrobiopterin in Animal Tissue Samples by HPLC with Electrochemical Detection—Protocol Optimization and Pitfalls. Antioxidants. 2022; 11(6):1182. https://doi.org/10.3390/antiox11061182
Chicago/Turabian StyleVujacic-Mirski, Ksenija, Matthias Oelze, Ivana Kuntic, Marin Kuntic, Sanela Kalinovic, Huige Li, Jacek Zielonka, Thomas Münzel, and Andreas Daiber. 2022. "Measurement of Tetrahydrobiopterin in Animal Tissue Samples by HPLC with Electrochemical Detection—Protocol Optimization and Pitfalls" Antioxidants 11, no. 6: 1182. https://doi.org/10.3390/antiox11061182