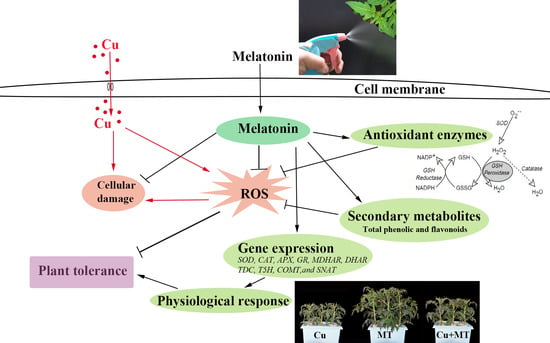
Melatonin Alleviates Copper Toxicity via Improving ROS Metabolism and Antioxidant Defense Response in Tomato Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Evaluation of Morphological and Photosynthesis Parameters
2.3. Transmission Electron Microscope (TEM) Analysis
2.4. Assessment of MDA, Relative Electrolyte Leakage and Histochemical Recognition of H2O2 and O2•−
2.5. Measurement of Enzymatic Antioxidant Compounds
2.6. Evaluation of Nonenzymatic Antioxidant Mixtures
2.7. Measurement of Cu and MT Contents in Shoot and Root
2.8. Virus-Induced Gene Silencing Constructs and Agrobacterium-Mediated Virus Infection
2.9. RNA Extraction and Gene Expression Assay via the Quantitative Real-Time PCR Approach
2.10. Statistical Analysis
3. Results
3.1. Influence of MT on the Growth of Tomato Plants Subjected to CS
3.2. Influence of MT on Ultrastructure Alterations in Mesophyll Cells and Chloroplasts of Tomato Leaves Subjected to CS
3.3. MT and Copper Content Modifications in MT and pCPA Treated Tomato Leaves
3.4. Exogenous MT Mitigates Oxidative Stress and Maintains Membrane Integrity
3.5. Influence of MT on Enzymatic Antioxidant Capacity in Tomato Leaves under CS
3.6. Effect of MT on Nonenzymatic and Total Antioxidant Activity Subjected to CS
3.7. Influence of Exogenous MT on Gene Expression Related to Detoxification and MT Biosynthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duruibe, J.O.; Ogwuegbu, M.; Egwurugwu, J. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, A.L. Copper as an essential for plant growth. Plant Physiol. 1931, 6, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Burkhead, J.L.; Gogolin, R.K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, F.; Zhang, F. Toxicity of copper and zinc in seedlings of mung bean and inducing accumulation of polyamine. J. Plant Nutr. 1998, 21, 1153–1162. [Google Scholar] [CrossRef]
- Benimeli, C.S.; Medina, A.; Navarro, C.M.; Medina, R.B.; Amoroso, M.J.; Gómez, M.I. Bioaccumulation of copper by Zea mays: Impact on root, shoot and leaf growth. Water Air Soil Pollut. 2010, 210, 365–370. [Google Scholar] [CrossRef]
- Mocquot, B.; Vangronsveld, J.; Clijsters, H.; Mench, M. Copper toxicity in young maize (Zea mays L.) plants: Effects on growth, mineral and chlorophyll contents, and enzyme activities. Plant Soil 1996, 182, 287–300. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W. Effect of Cu toxicity on growth of cowpea (Vigna unguiculata). Plant Soil 2006, 279, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Morris, H.; Cronin, M. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [Green Version]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Javed, M.T.; Akram, M.S.; Tanwir, K.; Chaudhary, H.J.; Ali, Q.; Stoltz, E.; Lindberg, S. Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotoxicol. Environ. Saf. 2017, 141, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yu, H.; Li, T.; Zhang, X. Influence of cadmium stress on root exudates of high cadmium accumulating rice line (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2018, 150, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Küpper, H.; Lombi, E.; Zhao, F.J.; McGrath, S.P. Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 2000, 212, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Shen, H.; Xu, L.; Zhu, X.; Li, C.; Zhang, W.; Xie, Y.; Gong, Y.; Liu, L. Transport, ultrastructural localization, and distribution of chemical forms of lead in radish (Raphanus sativus L.). Front. Plant Sci. 2015, 6, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.; Ahammed, G.J.; Yin, L.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J.; Zhou, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6, 601. [Google Scholar] [CrossRef]
- Anjum, N.A.; Hasanuzzaman, M.; Hossain, M.A.; Thangavel, P.; Roychoudhury, A.; Gill, S.S.; Rodrigo, M.A.M.; Adam, V.; Fujita, M.; Kizek, R. Jacks of metal/metalloid chelation trade in plants—An overview. Front. Plant Sci. 2015, 6, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Dubbels, R.; Reiter, R.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal. Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.S.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-induced water stress tolerance in plants: Recent advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Ding, F. Melatonin mitigates chilling-induced oxidative stress and photosynthesis inhibition in tomato plants. Antioxidants 2020, 9, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A. Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant 2021, 172, 820–846. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. The physiological function of melatonin in plants. Plant Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Latif Khan, A.; Shahzad, R.; Aaqil Khan, M.; Bilal, S.; Khan, A.; Kang, S.-M.; Lee, I.J. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants 2021, 13, plab026. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Hosseini, M.S.; Fahadi Hoveizeh, N.; Gholami, R.; Abdelrahman, M.; Tran, L.S.P. Exogenous melatonin mitigates salinity-induced damage in olive seedlings by modulating ion homeostasis, antioxidant defense, and phytohormone balance. Physiol. Plant. 2021, 173, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Zhang, H.; Tang, M. Exogenous melatonin application enhances Rhizophagus irregularis symbiosis and induces the antioxidant response of Medicago truncatula under lead stress. Front. Microbiol. 2020, 11, 516. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Qi, C.D.; Li, S.; Wang, Z.; Wang, X.; Wang, J.; Ren, S.; Li, X.; Zhang, N.; Guo, Y.D. Melatonin alleviates copper toxicity via improving copper sequestration and ros scavenging in Cucumber. Plant Cell Physiol. 2019, 60, 562–574. [Google Scholar] [CrossRef]
- Clemensson-Lindell, A. Triphenyltetrazolium chloride as an indicator of fine-root vitality and environmental stress in coniferous forest stands: Applications and limitations. Plant Soil 1994, 159, 297–300. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Analysis 1983, 603, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Daudi, A.; O’Brien, J.A. Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio-Protocol 2012, 2, e263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio-Protocol 2014, 4, e1108. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Ukeda, H.; Kawana, D.; Maeda, S.; Sawamura, M. Spectrophotometric assay for superoxide dismutase based on the reduction of highly water-soluble tetrazolium salts by xanthine-xanthine oxidase. Biosci. Biotech. Bioch. 1999, 63, 485–488. [Google Scholar] [CrossRef]
- Reuveni, R.; Shimoni, M.; Karchi, Z.; Kuc, J. Peroxidase activity as a biochemical marker for resistance of muskmelon (Cumcumis melo) to Pseudoperonospora cubensis. Phytopathology 1992, 82, 749–753. [Google Scholar] [CrossRef]
- Garcia, C.; Fedrigo, K.; Gabriel, A.; Botelho, R.V.; Rodrigues, J.D.; Ono, E.O. Control of mildew in vines with cinnamon extract and catalase activity in organic production. Res. Soc. Dev. 2021, 10, e214101018885. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Al-Saikhan, M.S.; Howard, L.R.; Miller, J.C., Jr. Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum, L.). J. Food Sci. 1995, 60, 341–343. [Google Scholar] [CrossRef]
- Jia, Z.S.; Tang, M.C.; Wu, J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Logan, B.A.; Demmig-Adams, B.; Adams III, W.W.; Grace, S.C. Antioxidants and xanthophyll cycle-dependent energy dissipation in Cucurbita pepo L. and Vinca major L. acclimated to four growth PPFDs in the field. J. Exp. Bot. 1998, 49, 1869–1879. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Analyt. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Pothinuch, P.; Tongchitpakdee, S. Melatonin contents in mulberry (Morus spp.) leaves: Effects of sample preparation, cultivar, leaf age and tea processing. Food Chem. 2011, 128, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Botany 2019, 161, 303–311. [Google Scholar] [CrossRef]
- Shen, J.L.; Wang, Y.; Shu, S.; Jahan, M.S.; Zhong, M.; Wu, J.Q.; Sun, J.; Guo, S.R. Exogenous putrescine regulates leaf starch overaccumulation in cucumber under salt stress. Sci. Hortic. 2019, 253, 99–110. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal. Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Azmitia, E.C. Evolution of serotonin: Sunlight to suicide. In Handbook of Behavioral Neuroscience; Müller, C.P., Jacobs, B.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 3–22. [Google Scholar]
- Esteban, M.; Cuesta, A.; Chaves-Pozo, E.; Meseguer, J. Influence of melatonin on the immune system of fish: A review. J. Mol. Sci. 2013, 14, 7979–7999. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Haldar, C.; Singh, R. Modulation of immunity in young-adult and aged squirrel, Funambulus pennanti by melatonin and p-chlorophenylalanine. Immun. Ageing 2009, 6, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byeon, Y.; Lee, H.Y.; Lee, K.; Back, K. Caffeic acid O-methyltransferase is involved in the synthesis of melatonin by methylating N-acetylserotonin in Arabidopsis. J. Pineal Res. 2014, 57, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Choi, G.-H.; Lee, H.Y.; Back, K. Melatonin biosynthesis requires N-acetylserotonin methyltransferase activity of caffeic acid O-methyltransferase in rice. J. Exp. Bot. 2015, 66, 6917–6925. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.Y.; Zhang, Y.; Xu, Y.P.; Qi, Z.Y.; Li, M.Q.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017, 62, e12387. [Google Scholar] [CrossRef]
- Öncel, I.; Keleş, Y.; Üstün, A. Interactive effects of temperature and heavy metal stress on the growth and some biochemical compounds in wheat seedlings. Environ. Pollut. 2000, 107, 315–320. [Google Scholar] [CrossRef]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int. J. Plant Prod. 2009, 3, 66–75. [Google Scholar]
- Ambrosini, V.G.; Rosa, D.J.; de Melo, G.W.B.; Zalamena, J.; Cella, C.; Simão, D.G.; da Silva, L.S.; Dos Santos, H.P.; Toselli, M.; Tiecher, T.L. High copper content in vineyard soils promotes modifications in photosynthetic parameters and morphological changes in the root system of ‘Red Niagara’plantlets. Plant Physiol. Bioch. 2018, 128, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Huang, Y.; Paglia, K.; Vaniya, A.; Wancewicz, B.; Keller, A.A. Metabolomics reveals the molecular mechanisms of copper induced cucumber leaf (Cucumis sativus) senescence. Environ. Sci. Technol. 2018, 52, 7092–7100. [Google Scholar] [CrossRef]
- Rudolph, A.S.; Crowe, J.H. Membrane stabilization during freezing: The role of two natural cryoprotectants, trehalose and proline. Cryobiology 1985, 22, 367–377. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, R.; Meng, J.; Li, Z.; Wu, Y.; Tao, J. Ameliorative effects of melatonin on dark-induced leaf senescence in gardenia (Gardenia jasminoides Ellis): Leaf morphology, anatomy, physiology and transcriptome. Sci. Rep. 2017, 7, 10423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Q.; Li, G.; Cui, Z.; Yang, F.; Jiang, X.; Diallo, L.; Kong, F. Seed priming with melatonin improves the seed germination of waxy maize under chilling stress via promoting the antioxidant system and starch metabolism. Sci. Rep. 2019, 9, 15044. [Google Scholar] [CrossRef] [Green Version]
- Thounaojam, T.C.; Panda, P.; Mazumdar, P.; Kumar, D.; Sharma, G.D.; Sahoo, L.; Sanjib, P. Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol. Bioch. 2012, 53, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ghori, N.-H.; Ghori, T.; Hayat, M.; Imadi, S.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Tech. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Inzé, D.; Van Montagu, M. Oxidative stress in plants. Curr. Opin. Biotech. 1995, 6, 153–158. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Manara, A. Plant responses to heavy metal toxicity. In Plants and Heavy Metals; Furini, A., Ed.; Springer: Dordrecht, Switzerland, 2012; pp. 27–53. [Google Scholar]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K. Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Li, M.; Liu, S.; Pan, T.; Ma, C.; Qiu, D. Melatonin-mediate acid rain stress tolerance mechanism through alteration of transcriptional factors and secondary metabolites gene expression in tomato. Ecotoxicol. Environ. Saf. 2020, 200, 110720. [Google Scholar] [CrossRef]
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. based DPPH assay for antioxidant activity analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Saroyo, H.; Arifah, I.A.N. Antioxidant activity using DPPH & FRAP method and their correlation with the levels of phenolic and flavonoid compounds from nemba plants (Azadirachta Indica A. Juss). J. Nutraceuticals Herb. Med. 2021, 3, 10–20. [Google Scholar]
- Szalai, G.; Kellős, T.; Galiba, G.; Kocsy, G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J. Plant Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- Sahin, G.; De Tullio, M.C. A winning two pair: Role of the redox pairs AsA/DHA and GSH/GSSG in signal transduction. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Anjum, N., Chan, M.-T., Umar, S., Eds.; Springer: Dordrecht, Switzerland, 2010; pp. 251–263. [Google Scholar]
- Miret, J.A.; Müller, M. AsA/DHA redox pair influencing plant growth and stress tolerance. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M., Munné-Bosch, S., Burritt, D., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 297–319. [Google Scholar]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020, 399, 123020. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.; Corpas, F.J.; López-Huertas, E.; Palma, J.M. Plant superoxide dismutases: Function under abiotic stress conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar]







| Treatment Parameter | CK | Cu | MT | Cu + MT | Cu + pCPA |
|---|---|---|---|---|---|
| Plant height (cm) | 17.71 ± 0.49b | 11.62 ± 0.30c | 22.08 ± 0.60a | 15.45 ± 0.14d | 7.84 ± 0.25e |
| Shoot Fresh Weight (g/plant) | 15.92 ± 0.79b | 9.33 ± 0.37d | 26.25 ± 1.38a | 13.04 ± 0.80c | 5.58 ± 0.33e |
| Root Fresh Weight (g/plant) | 3.24 ± 0.13b | 2.44 ± 0.10c | 4.71 ± 0.41a | 4.61 ± 0.27a | 1.57 ± 0.10d |
| Shoot Dry Weight (g/plant) | 1.45 ± 0.06b | 1.07 ± 0.02c | 1.92 ± 0.08a | 1.41 ± 0.03b | 0.61 ± 0.01d |
| Root Dry Weight (g/plant) | 0.37 ± 0.01c | 0.32 ± 0.01d | 0.52 ± 0.01a | 0.44 ± 0.01b | 0.2 ± 0.01e |
| TTC [ug/(g·h)] | 4547.92 ± 150.91b | 170.56 ± 20.41c | 7225.19 ± 401.94a | 733.95 ± 27.75c | 151.23 ± 8.96c |
| Total Chlorophyll (Ca + Cb) (mg/g·FW) | 1.77 ± 0.12b | 0.80 ± 0.03d | 2.07 ± 0.08a | 1.43 ± 0.05c | 0.74 ± 0.10d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Wang, Y.; Ma, X.; Ouyang, Z.; Deng, L.; Shen, S.; Dong, X.; Du, N.; Dong, H.; Guo, Z.; et al. Melatonin Alleviates Copper Toxicity via Improving ROS Metabolism and Antioxidant Defense Response in Tomato Seedlings. Antioxidants 2022, 11, 758. https://doi.org/10.3390/antiox11040758
Zhang T, Wang Y, Ma X, Ouyang Z, Deng L, Shen S, Dong X, Du N, Dong H, Guo Z, et al. Melatonin Alleviates Copper Toxicity via Improving ROS Metabolism and Antioxidant Defense Response in Tomato Seedlings. Antioxidants. 2022; 11(4):758. https://doi.org/10.3390/antiox11040758
Chicago/Turabian StyleZhang, Tao, Yong Wang, Xiaojing Ma, Zhaopeng Ouyang, Lei Deng, Shunshan Shen, Xiaoxing Dong, Nanshan Du, Han Dong, Zhixin Guo, and et al. 2022. "Melatonin Alleviates Copper Toxicity via Improving ROS Metabolism and Antioxidant Defense Response in Tomato Seedlings" Antioxidants 11, no. 4: 758. https://doi.org/10.3390/antiox11040758