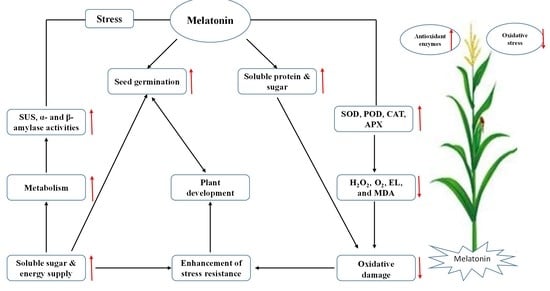
Melatonin Application Alleviates Stress-Induced Photosynthetic Inhibition and Oxidative Damage by Regulating Antioxidant Defense System of Maize: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database Construction and Literature Search
2.2. Data Calculation and Analysis
3. Results
3.1. Publication Bias and Data Heterogeneity
3.2. Exogenous Melatonin Improved Biomass and Plant Growth
3.3. Exogenous Melatonin Improved Enzymatic Activities
3.4. Exogenous Melatonin Effect Proline and Reduced the Malonaldehyde, Superoxide, and Hydrogen Peroxide
3.5. Exogenous Melatonin Stimulated Soluble Sugar and Protein, and Reduced Electrolyte Leakage
3.6. Exogenous Melatonin Impacts Gas Exchange Parameters
3.7. Exogenous Melatonin Improved Leaf Chlorophyll Content
3.8. Effect of Exogenous Melatonin on Leaf Relative Water Content, Leaf Relative Water Potential, and Water Use Efficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, L.; Huang, Z.; Li, S.; Ashraf, U.; Yang, W.; Liu, H.; Xu, D.; Li, W.; Mo, Z. Melatonin and nitrogen applications modulate early growth and related physio-biochemical attributes in maize under Cd stress. J. Soil Sci. Plant Nutr. 2021, 21, 978–990. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Zhao, C.; Xue, J.; Zhang, R. Exogenous melatonin improves drought tolerance in maize seedlings by regulating photosynthesis and the ascorbate–glutathione cycle. Russ. J. Plant Physiol. 2020, 67, 809–821. [Google Scholar] [CrossRef]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B.; Gu, Y.; Liao, J.Q.; Hu, C.; Zhang, Z.W.; Yuan, S. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Ali, S.; Rizwan, M.; Hussain, M.B.; Hussain, A.; Alam, P.; Alqarawi, A.A.; Hashem, A.; AbdAllah, E.F. The ameliorative role of 5-aminolevulinic acid (ALA) under Cr stress in two maize cultivars showing differential sensitivity to Cr stress tolerance. J. Plant Growth Regul. 2019, 38, 788–798. [Google Scholar] [CrossRef]
- Lv, S.; Yang, X.; Lin, X.; Liu, Z.; Zhao, J.; Li, K.; Mu, C.; Chen, X.; Chen, F.; Mi, G. Yield gap simulations using ten maize cultivars commonly planted in Northeast China during the past five decades. Agric. For. Meteorol. 2015, 205, 1–10. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Zia-ur-Rehman, M.; Abbas, Z.; Hannan, F. Use of maize (Zea mays L.) for phytomanagement of Cd-contaminated soils: A critical review. Environ. Geochem. Health 2017, 39, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Sürücü, A.; Mohammad, D.M.; Günal, E.; Budak, M. Concentration of heavy metals in soils along three major roads of Sulaimani, Northeast Iraq. Carpathian J. Earth Environ. Sci. 2018, 13, 523–538. [Google Scholar] [CrossRef]
- Okant, M.; Kaya, C. The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ. Sci. Pollut. Res. 2019, 26, 11864–11874. [Google Scholar] [CrossRef]
- Cao, Q.; Li, G.; Cui, Z.; Yang, F.; Jiang, X.; Diallo, L.; Kong, F. Seed priming with melatonin improves the seed germination of waxy maize under chilling stress via promoting the antioxidant system and starch metabolism. Sci. Rep. 2019, 9, 15044. [Google Scholar] [CrossRef] [Green Version]
- Turk, H.; Genisel, M. Melatonin-related mitochondrial respiration responses are associated with growth promotion and cold tolerance in plants. Cryobiology 2020, 92, 76–85. [Google Scholar] [CrossRef]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pál, M.; Horváth, E.; Janda, T.; Páldi, E.; Szalai, G. Physiological changes and defense mechanisms induced by cadmium stress in maize. J. Plant Nutr. Soil Sci. 2006, 169, 239–246. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, T.; Zia-ur-Rehman, M.; Naeem, A.; Nawaz, R.; Ali, S.; Murtaza, G.; Maqsood, M.A.; Azhar, M.; Khalid, H.; Rizwan, M. Photosynthesis and growth response of maize (Zea mays L.) hybrids exposed to cadmium stress. Environ. Sci. Pollut. Res. 2017, 24, 5521–5529. [Google Scholar] [CrossRef]
- Ren, S.; Deng, Q.; Peng, J.; Lin, L.; Zhang, H. Effects of exogenous melatonin on growth and cadmium content of Zizyphus acidojujuba seedlings. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; p. 042006. [Google Scholar]
- Ahmad, S.; Kamran, M.; Zhou, X.; Ahmad, I.; Meng, X.; Javed, T.; Iqbal, A.; Wang, G.; Su, W.; Wu, X. Melatonin improves the seed filling rate and endogenous hormonal mechanism in grains of summer maize. Physiol. Plant. 2021, 172, 1059–1072. [Google Scholar] [CrossRef]
- Khan, A.; Zahir Afridi, M.; Airf, M.; Ali, S.; Muhammad, I. A sustainable approach toward maize production: Effectiveness of farm yard manure and urea N. Ann. Biol. Sci. 2017, 5, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Lv, X.; Li, T.; Wen, X.; Liao, Y.; Liu, Y. Effect of potassium foliage application post-anthesis on grain filling of wheat under drought stress. Field Crops Res. 2017, 206, 95–105. [Google Scholar] [CrossRef]
- Kamran, M.; Wennan, S.; Ahmad, I.; Xiangping, M.; Wenwen, C.; Xudong, Z.; Siwei, M.; Khan, A.; Qingfang, H.; Tiening, L. Application of paclobutrazol affect maize grain yield by regulating root morphological and physiological characteristics under a semi-arid region. Sci. Rep. 2018, 8, 4818. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Ren, J.; Yin, L.; Liu, Y.; Deng, X.; Liu, P.; Wang, S. Exogenous melatonin alleviates PEG-induced short-term water deficiency in maize by increasing hydraulic conductance. BMC Plant Biol. 2020, 20, 218. [Google Scholar] [CrossRef]
- Li, Z.; Su, X.; Chen, Y.; Fan, X.; He, L.; Guo, J.; Wang, Y.; Yang, Q. Melatonin improves drought resistance in maize seedlings by enhancing the antioxidant system and regulating abscisic acid metabolism to maintain stomatal opening under PEG-induced drought. J. Plant Biol. 2021, 64, 299–312. [Google Scholar] [CrossRef]
- Dubey, S.; Bhargava, A.; Fuentes, F.; Shukla, S.; Srivastava, S. Effect of salinity stress on yield and quality parameters in flax (Linum usitatissimum L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 954–966. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Song, X. Seed priming with melatonin effects on seed germination and seedling growth in maize under salinity stress. Pak. J. Bot. 2016, 48, 1345–1352. [Google Scholar]
- Velmurugan, A.; Swarnam, P.; Subramani, T.; Meena, B.; Kaledhonkar, M. Water demand and salinity. In Desalination-Challenges and Opportunities; IntechOpen: London, UK, 2020. [Google Scholar]
- Rajabi Dehnavi, A.; Zahedi, M.; Ludwiczak, A.; Cardenas Perez, S.; Piernik, A. Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- Chi, Y.X.; Gao, F.; Muhammad, I.; Huang, J.H.; Zhou, X.B. Effect of water conditions and nitrogen application on maize growth, carbon accumulation and metabolism of maize plant in subtropical regions. Arch. Agron. Soil Sci. 2022, 1–15. [Google Scholar] [CrossRef]
- Ghosh, N.; Adak, M.; Ghosh, P.; Gupta, S.; Gupta, D.S.; Mandal, C. Differential responses of two rice varieties to salt stress. Plant Biotechnol. Rep. 2011, 5, 89–103. [Google Scholar] [CrossRef]
- Abbasi, G.H.; Akhtar, J.; Ahmad, R.; Jamil, M.; Anwar-ul-Haq, M.; Ali, S.; Ijaz, M. Potassium application mitigates salt stress differentially at different growth stages in tolerant and sensitive maize hybrids. Plant Growth Regul. 2015, 76, 111–125. [Google Scholar] [CrossRef]
- Sezer, İ.; Kiremit, M.S.; Öztürk, E.; Subrata, B.A.G.; Osman, H.M.; Akay, H.; Arslan, H. Role of melatonin in improving leaf mineral content and growth of sweet corn seedlings under different soil salinity levels. Sci. Hortic. 2021, 288, 110376. [Google Scholar] [CrossRef]
- Wang, D.Y.; Wang, J.; Shi, S.H.; Huang, L.X.; Zhu, M.; Li, F.H. Exogenous melatonin ameliorates salinity-induced oxidative stress and improves photosynthetic capacity in sweet corn seedlings. Photosynthetica 2021, 59, 327–336. [Google Scholar] [CrossRef]
- Kurunc, A.; Aslan, G.E.; Karaca, C.; Tezcan, A.; Turgut, K.; Karhan, M.; Kaplan, B. Effects of salt source and irrigation water salinity on growth, yield and quality parameters of Stevia rebaudiana Bertoni. Sci. Hortic. 2020, 270, 109458. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13, e0202175. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, N.; Ghassemi-Golezani, K. Physiological changes of Mentha pulegium in response to exogenous salicylic acid under salinity. Sci. Hortic. 2020, 267, 109325. [Google Scholar] [CrossRef]
- Youssef, N.M.; Hashish, K.I.; Taha, L.S. Salinity tolerance improvement of in vitro propagated Paulownia tomentosa using proline. Bull. Natl. Res. Cent. 2020, 44, 90. [Google Scholar] [CrossRef]
- Bowes, K.; Mercuri, A.M.; Rattighieri, E.; Rinaldi, R.; Arnoldus-Huyzendveld, A.; Ghisleni, M.; Grey, C.; Mackinnon, M.; Vaccaro, E. Palaeoenvironment and land use of Roman peasant farmhouses in southern Tuscany. Plant Biosyst. 2015, 149, 174–184. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, Y.; He, H.; Xi, Q.; Fu, J.; Zhao, Y. Exogenous melatonin improves growth in hulless barley seedlings under cold stress by influencing the expression rhythms of circadian clock genes. PeerJ 2021, 9, e10740. [Google Scholar] [CrossRef]
- Kul, R.; Esringü, A.; Dadasoglu, E.; Sahin, Ü.; Turan, M.; Örs, S.; Ekinci, M.; Agar, G.; Yildirim, E. Melatonin: Role in increasing plant tolerance in abiotic stress conditions. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019; pp. 109–128. [Google Scholar]
- Ren, J.; Ye, J.; Yin, L.; Li, G.; Deng, X.; Wang, S. Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Yan, F.; Wei, H.; Li, W.; Liu, Z.; Tang, S.; Chen, L.; Ding, C.; Jiang, Y.; Ding, Y.; Li, G. Melatonin improves K+ and Na+ homeostasis in rice under salt stress by mediated nitric oxide. Ecotoxicol. Environ. Saf. 2020, 206, 111358. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Cui, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Su, W.; Javed, T.; El-Serehy, H.A.; Jia, Z.; et al. Exogenous application of melatonin induces tolerance to salt stress by improving the photosynthetic efficiency and antioxidant defense system of maize seedling. J. Plant Growth Regul. 2021, 40, 1270–1283. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, X.; Niu, J. Effects of melatonin on morphological characteristics, mineral nutrition, nitrogen metabolism, and energy status in alfalfa under high-nitrate stress. Front. Plant Sci. 2021, 12, 694179. [Google Scholar] [CrossRef]
- Muhammad, I.; Sainju, U.M.; Zhao, F.; Khan, A.; Ghimire, R.; Fu, X.; Wang, J. Regulation of soil CO2 and N2O emissions by cover crops: A meta-analysis. Soil Till. Res. 2019, 192, 103–112. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, J.; Sainju, U.M.; Zhang, S.; Zhao, F.; Khan, A. Cover cropping enhances soil microbial biomass and affects microbial community structure: A meta-analysis. Geoderma 2021, 381, 114696. [Google Scholar] [CrossRef]
- Huedo-Medina, T.B.; Sánchez-Meca, J.; Marin-Martinez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I² index? Psychol. Methods 2006, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Dieleman, W.I.; Janssens, I.A. Can publication bias affect ecological research? A case study on soil respiration under elevated CO2. New Phytol. 2011, 190, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Wang, J.; Khan, A.; Ahmad, S.; Yang, L.; Ali, I.; Zeeshan, M.; Ullah, S.; Fahad, S.; Ali, S.; et al. Impact of the mixture verses solo residue management and climatic conditions on soil microbial biomass carbon to nitrogen ratio: A systematic review. Environ. Sci. Pollut. Res. 2021, 28, 64241–64252. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh, R.; Meysam, H.S. Effect of different levels of drought stress (PEG 6000 concentrations) on seed germination and inorganic elements content in purslane (Portulaca oleraceae L.) leaves. J. Stress Physiol. Biochem. 2012, 8, 51–61. [Google Scholar]
- Deng, B.L.; Yang, K.J.; Zhang, Y.F.; Li, Z.T. Can antioxidant’s reactive oxygen species (ROS) scavenging capacity contribute to aged seed recovery? Contrasting effect of melatonin, ascorbate and glutathione on germination ability of aged maize seeds. Free Radic. Res. 2017, 51, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Erdal, S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Boga, J.A.; Caballero, B.; Potes, Y.; Perez-Martinez, Z.; Reiter, R.J.; Vega-Naredo, I.; Coto-Montes, A. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 2019, 66, e12534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Wang, M.; Duan, S.; Zhou, Z.; Chen, S.; Wang, D. Foliar spraying of melatonin confers cadmium tolerance in Nicotiana tabacum L. Ecotoxicol. Environ. Saf. 2019, 170, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; Ma, F. Unveiling the mechanism of melatonin impacts on maize seedling growth: Sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (C-ucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Bhatla, S.C. Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radic. Biol. Med. 2017, 106, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Islam, F.; Yang, C.; Nawaz, A.; Athar, H.-u.-R.; Gill, R.A.; Ali, B.; Song, W.; Zhou, W. Methyl jasmonate alleviates arsenic-induced oxidative damage and modulates the ascorbate–glutathione cycle in oilseed rape roots. Plant Growth Regul. 2018, 84, 135–148. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef]
- Lin, L.; Li, J.; Chen, F.; Liao, M.a.; Tang, Y.; Liang, D.; Xia, H.; Lai, Y.; Wang, X.; Chen, C. Effects of melatonin on the growth and cadmium characteristics of Cyphomandra betacea seedlings. Environ. Monit. Assess. 2018, 190, 119. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Khan, I.; Raza, M.A.; Awan, S.A.; Shah, G.A.; Rizwan, M.; Ali, B.; Tariq, R.; Hassan, M.J.; Alyemeni, M.N.; Brestic, M. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): The oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol. Biochem. 2020, 156, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Marta, B.; Szafrańska, K.; Posmyk, M.M. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 2016, 7, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Zeeshan, M.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative effect of melatonin improves drought tolerance by regulating growth, photosynthetic traits and leaf ultrastructure of maize seedlings. BMC Plant Biol. 2021, 21, 368. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Wang, W.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Lee, G.; Carrow, R.N.; Duncan, R.R.; Eiteman, M.A.; Rieger, M.W. Synthesis of organic osmolytes and salt tolerance mechanisms in Paspalum vaginatum. Environ. Exp. Bot. 2008, 63, 19–27. [Google Scholar] [CrossRef]
- Chen, Y.E.; Liu, W.J.; Su, Y.Q.; Cui, J.M.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physiol. Plant. 2016, 158, 225–235. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, H.; Cao, K.; Hu, L.; Du, T.; Baluška, F.; Zou, Z. Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front. Plant Sci. 2016, 7, 1823. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem ii in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef] [Green Version]
- Gleason, S.M.; Wiggans, D.R.; Bliss, C.A.; Comas, L.H.; Cooper, M.; DeJonge, K.C.; Young, J.S.; Zhang, H. Coordinated decline in photosynthesis and hydraulic conductance during drought stress in Zea mays. Flora 2017, 227, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.N.; Avila, R.G.; de Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Ahmadi, J.; Mehrabi, A.A.; Etminan, A.; Moghaddam, M.; Siddique, K.H. Physiological responses to drought stress in wild relatives of wheat: Implications for wheat improvement. Acta Physiol. Plant. 2017, 39, 106. [Google Scholar] [CrossRef]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in M edicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Wang, T.; Wang, P. Mitigation effect and mechanism of exogenous melatonin on maize seedling under salt stress. Chin. J. Grassl. 2020, 42, 14–21. [Google Scholar]
- Cao, L.; Kou, F.; Zhang, M.; Jin, X.; Ren, C.; Yu, G.; Zhang, Y.; Wang, M. Effect of exogenous melatonin on the quality of soybean and natto products under drought stress. J. Chem. 2021, 2021, 8847698. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Zeeshan, M.; Zhou, X.B. Melatonin and KNO3 Application improves growth, physiological and biochemical characteristics of maize seedlings under waterlogging stress conditions. Biology 2022, 11, 99. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Chi, Y.X.; Zeeshan, M.; Nasar, J.; Zhou, X.B. Interactive effects of melatonin and nitrogen improve drought tolerance of maize seedlings by regulating growth and physiochemical attributes. Antioxidants 2022, 11, 359. [Google Scholar] [CrossRef]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Role of melatonin in alleviating cold stress in A rabidopsis thaliana. J. Pineal Res. 2014, 56, 238–245. [Google Scholar] [CrossRef]
- Chi, Y.X.; Yang, L.; Zhao, C.J.; Muhammad, I.; Zhou, X.B.; De Zhu, H. Effects of soaking seeds in exogenous vitamins on active oxygen metabolism and seedling growth under low-temperature stress. Saudi J. Biol. Sci. 2021, 28, 3254–3261. [Google Scholar]
- Moghadam, N.K.; Motesharezadeh, B.; Maali-Amiri, R.; Lajayer, B.A.; Astatkie, T. Effects of potassium and zinc on physiology and chlorophyll fluorescence of two cultivars of canola grown under salinity stress. Arab. J. Geosci. 2020, 13, 771. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.G.; Tan, D.X. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef] [Green Version]







| Variables | Photosynthetic Rate | Stomatal Conductance | Transpiration Rate | Intercellular CO2 Concentration | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| lnRR++ | Bootstrap CI | N | lnRR++ | Bootstrap CI | N | lnRR++ | Bootstrap CI | N | lnRR++ | Bootstrap CI | N | |||||
| Overall | 0.1731 | 0.1340 | 0.2120 | 95 | 0.1703 | 0.1231 | 0.2187 | 83 | 0.2145 | 0.1596 | 0.2701 | 63 | −0.0240 | −0.0834 | 0.0389 | 12 |
| Stress types | ||||||||||||||||
| No stress | 0.0957 | 0.0607 | 0.1310 | 54 | 0.0263 | −0.0330 | 0.0798 | 33 | 0.0194 | −0.0034 | 0.0465 | 22 | −0.0085 | −0.1013 | 0.0725 | 5 |
| Drought stress | 0.2705 | 0.1919 | 0.3599 | 33 | 0.2627 | 0.1843 | 0.3460 | 33 | 0.3167 | 0.2404 | 0.4028 | 33 | −0.0230 | −0.0727 | 0.0311 | 4 |
| Salt stress | 0.2666 | 0.1893 | 0.3910 | 8 | 0.2618 | 0.2183 | 0.3058 | 17 | 0.3556 | 0.2689 | 0.4436 | 8 | −0.0573 | −0.1979 | 0.3435 | 3 |
| Study types | ||||||||||||||||
| Pot | 0.1822 | 0.1230 | 0.2478 | 53 | 0.1788 | 0.1258 | 0.2338 | 65 | 0.2225 | 0.1546 | 0.2953 | 45 | 0.0097 | −0.0419 | 0.0530 | 6 |
| Growth chamber | 0.1325 | 0.0494 | 0.2194 | 8 | 0.1516 | 0.0540 | 0.2462 | 8 | 0.1382 | 0.0319 | 0.2574 | 8 | −0.0663 | −0.1657 | 0.0428 | 4 |
| Hydroponic | 0.1274 | −0.0177 | 0.3193 | 10 | 0.135 | 0.0402 | 0.2398 | 10 | 0.2598 | 0.1079 | 0.4218 | 10 | −0.0683 | −0.1878 | 0.3435 | 2 |
| Field | 0.1879 | 0.1500 | 0.2247 | 24 | ||||||||||||
| Maize varieties | ||||||||||||||||
| Zhengdan 958 | 0.1949 | 0.1595 | 0.2337 | 50 | 0.1858 | 0.1340 | 0.2431 | 26 | 0.2522 | 0.1728 | 0.3292 | 26 | ||||
| Others | 0.2079 | 0.0590 | 0.3664 | 20 | 0.1841 | 0.0365 | 0.3329 | 20 | 0.3290 | 0.1598 | 0.5327 | 12 | −0.0021 | −0.0769 | 0.0742 | 8 |
| Azam | −0.1401 | −0.1643 | −0.0953 | 3 | −0.1539 | −0.2029 | −0.1076 | 3 | −0.0179 | −0.0535 | 0.0001 | 3 | ||||
| Mallika | 0.1544 | 0.1014 | 0.2124 | 3 | 0.0342 | 0.00001 | 0.0606 | 3 | 0.0425 | 0.0343 | 0.0477 | 3 | ||||
| Cheng Yu 888 | 0.0800 | 0.0260 | 0.1425 | 14 | 0.0887 | −0.0164 | 0.1838 | 14 | 0.1084 | 0.0286 | 0.2024 | 14 | −0.065 | −0.1665 | 0.0402 | 4 |
| Wanchuan 1306 | 0.2434 | 0.1488 | 0.3461 | 3 | 0.2618 | 0.1476 | 0.3482 | 3 | 0.3408 | 0.2368 | 0.4239 | 3 | ||||
| Shaanke 9 | 0.4368 | 0.0011 | 0.9240 | 3 | 0.3954 | 0.0124 | 0.8503 | 12 | 0.3490 | −0.0115 | 0.7173 | 2 | ||||
| Vega F1 | 0.2456 | 0.2094 | 0.2786 | 2 | ||||||||||||
| Variables | Leaf Chlorophyll Content | Chlorophyll a Content | Chlorophyll b Content | Carotenoid | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| lnRR++ | Bootstrap CI | N | lnRR++ | Bootstrap CI | N | lnRR++ | Bootstrap CI | N | lnRR++ | Bootstrap CI | N | |||||
| Overall | 0.1800 | 0.1352 | 0.2210 | 83 | 0.3826 | 0.2796 | 0.5030 | 29 | 0.2400 | 0.1567 | 0.3251 | 29 | 0.1813 | 0.0925 | 0.2619 | 24 |
| Stress types | ||||||||||||||||
| No stress | 0.1729 | 0.1301 | 0.2190 | 52 | 0.0382 | −0.0172 | 0.1237 | 9 | 0.0215 | −0.0402 | 0.1042 | 9 | 0.0050 | −0.0455 | 0.0755 | 6 |
| Drought stress | 0.3080 | 0.0980 | 0.4846 | 11 | 0.6257 | 0.4711 | 0.7673 | 12 | 0.4087 | 0.2909 | 0.5199 | 12 | 0.2802 | 0.1685 | 0.3910 | 15 |
| Salt stress | 0.1883 | 0.0907 | 0.3045 | 4 | 0.5198 | 0.3970 | 0.6408 | 4 | 0.3250 | 0.2397 | 0.3785 | 4 | 0.0805 | −0.0336 | 0.1587 | 3 |
| Lead (Pb) | 0.1149 | 0.0931 | 0.1366 | 2 | 0.3465 | 0.1421 | 0.5422 | 4 | 0.1707 | 0.0321 | 0.3049 | 4 | ||||
| Chilling stress | 0.1870 | 0.1106 | 0.3503 | 8 | ||||||||||||
| Cu stress | −0.0165 | −0.1522 | 0.0797 | 6 | ||||||||||||
| Study types | ||||||||||||||||
| Pot | 0.1386 | 0.0567 | 0.2287 | 28 | 0.4167 | 0.2974 | 0.5447 | 25 | 0.2424 | 0.1488 | 0.3298 | 25 | 0.1886 | 0.0936 | 0.2792 | 22 |
| Growth chamber | 0.0754 | 0.0130 | 0.1333 | 24 | ||||||||||||
| Hydroponic | 0.1935 | 0.1084 | 0.2680 | 7 | 0.2077 | 0.0869 | 0.3348 | 4 | 0.2328 | 0.0611 | 0.3438 | 4 | 0.0212 | 0.0134 | 0.0290 | 2 |
| Field | 0.2757 | 0.2218 | 0.3285 | 24 | ||||||||||||
| Maize varieties | ||||||||||||||||
| Zhengdan 958 | 0.2640 | 0.2092 | 0.3107 | 26 | 0.4433 | 0.2728 | 0.6132 | 16 | 0.2773 | 0.1512 | 0.4092 | 16 | 0.2086 | 0.0989 | 0.3178 | 16 |
| Others | 0.1099 | 0.0558 | 0.1686 | 48 | 0.3265 | 0.2944 | 0.3603 | 2 | 0.3371 | 0.3083 | 0.3683 | 2 | −0.0352 | −0.1792 | 0.0651 | 5 |
| BDK5783 | 0.0683 | 0.0229 | 0.1149 | 4 | 0.1888 | 0.0531 | 0.3470 | 8 | 0.0968 | 0.0217 | 0.1906 | 8 | ||||
| Wanchuan 1306 | 0.5892 | 0.4276 | 0.7353 | 3 | 0.6506 | 0.4892 | 0.7857 | 3 | 0.3666 | 0.2020 | 0.5048 | 3 | 0.3072 | 0.1951 | 0.4978 | 3 |
| Variables | Relative Water Content | Leaf Water Potential | Water Use Efficiency | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LnRR++ | Bootstrap CI | N | LnRR++ | Bootstrap CI | N | LnRR++ | Bootstrap CI | N | ||||
| Overall | 0.0699 | 0.0391 | 0.1025 | 60 | 0.0016 | −0.0405 | 0.0449 | 14 | −0.024 | −0.0834 | 0.0389 | 12 |
| Stress types | ||||||||||||
| No stress | −0.0023 | −0.0126 | 0.0066 | 22 | 0.0067 | −0.0296 | 0.0301 | 8 | −0.0085 | −0.1013 | 0.0725 | 5 |
| Drought stress | 0.1144 | 0.0537 | 0.1825 | 28 | −0.1116 | −0.1765 | −0.0603 | 2 | −0.023 | −0.0727 | 0.0311 | 4 |
| Salt stress | 0.111 | 0.0823 | 0.1459 | 9 | −0.0169 | −0.0545 | 0.0548 | 2 | −0.0573 | −0.1979 | 0.3435 | 3 |
| Lead (Pb) | 0.1149 | 0.0931 | 0.1366 | 2 | ||||||||
| Study types | ||||||||||||
| Pot | 0.0822 | 0.0377 | 0.1351 | 40 | 0.0208 | −0.0262 | 0.0676 | 8 | 0.0097 | −0.0419 | 0.053 | 6 |
| Growth chamber | 0.0456 | 0.0252 | 0.0662 | 20 | −0.0364 | −0.1166 | 0.0325 | 6 | −0.0663 | −0.1657 | 0.0428 | 4 |
| Hydroponic | −0.0683 | −0.1878 | 0.3435 | 2 | ||||||||
| Maize varieties | ||||||||||||
| Zhengdan 958 | 0.091 | 0.0514 | 0.1303 | 24 | −0.0731 | −0.1765 | 0.0337 | 2 | ||||
| Others | 0.1267 | 0.0345 | 0.2494 | 15 | −0.0021 | −0.0769 | 0.0742 | 8 | ||||
| BDK5783 | 0.0673 | 0.0229 | 0.1203 | 4 | ||||||||
| Cheng Yu 888 | 0.0246 | 0.0031 | 0.0477 | 14 | −0.0179 | −0.0557 | 0.0185 | 8 | −0.065 | −0.1665 | 0.0402 | 4 |
| Nonghua101 | 0.0534 | 0.0283 | 0.0784 | 2 | ||||||||
| Wanchuan 1306 | −0.1276 | −0.1865 | −0.0796 | 3 | ||||||||
| Hido | 0.0434 | 0.0111 | 0.0746 | 2 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, I.; Yang, L.; Ahmad, S.; Mosaad, I.S.M.; Al-Ghamdi, A.A.; Abbasi, A.M.; Zhou, X.-B. Melatonin Application Alleviates Stress-Induced Photosynthetic Inhibition and Oxidative Damage by Regulating Antioxidant Defense System of Maize: A Meta-Analysis. Antioxidants 2022, 11, 512. https://doi.org/10.3390/antiox11030512
Muhammad I, Yang L, Ahmad S, Mosaad ISM, Al-Ghamdi AA, Abbasi AM, Zhou X-B. Melatonin Application Alleviates Stress-Induced Photosynthetic Inhibition and Oxidative Damage by Regulating Antioxidant Defense System of Maize: A Meta-Analysis. Antioxidants. 2022; 11(3):512. https://doi.org/10.3390/antiox11030512
Chicago/Turabian StyleMuhammad, Ihsan, Li Yang, Shakeel Ahmad, Ibrahim S. M. Mosaad, Abdullah Ahmed Al-Ghamdi, Arshad Mehmood Abbasi, and Xun-Bo Zhou. 2022. "Melatonin Application Alleviates Stress-Induced Photosynthetic Inhibition and Oxidative Damage by Regulating Antioxidant Defense System of Maize: A Meta-Analysis" Antioxidants 11, no. 3: 512. https://doi.org/10.3390/antiox11030512