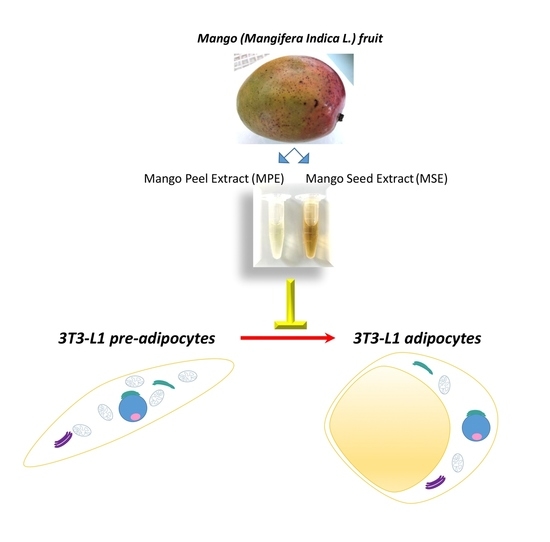
Bio-Waste Products of Mangifera indica L. Reduce Adipogenesis and Exert Antioxidant Effects on 3T3-L1 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Mango Peel and Seed Extracts
2.2. HPLC-ESI-MS Analysis
2.3. Cell Culture and Reagents
2.4. Adipocyte Differentiation and Treatments
2.5. Cell Viability Assay
2.6. Antioxidant Activity
2.7. Western Blot Analysis
2.8. Oil Red O (ORO) Staining
2.9. Detection of Reactive Oxygen Species Generation
2.10. Triacylglycerol Accumulation Assay
2.11. Statistical Analysis
3. Results
3.1. MPE and MSE Possess ROS Scavenger Activities
3.2. Effects of MPE and MSE on the Viability of 3T3-L1 Pre-Adipocytes
3.3. MPE and MSE Reduced Lipid Content during 3T3-L1 Adipocyte Differentiation
3.4. MPE and MSE Reduced the Expression of Key Factors of Adipogenic Differentiation and Lipid Accumulation
3.5. MPE and MSE Increase the Levels of Lipolytic Factors
3.6. MPE and MSE Reduce ROS Production in Adipocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | acetyl-CoA carboxylase |
| ACL | ATP-citrate lyase |
| AMPK | AMP-activated protein kinase |
| C/EBPα | CCAAT enhancer binding protein alpha |
| Compound C | CC |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl radical |
| FABP4/aP2 | adipocyte fatty acid-binding protein 4/adipocyte protein 2 |
| FAS | fatty acid synthase |
| FBS | fetal calf serum |
| GLUT4 | glucose transporter 4 |
| GPAT | glycerol-3-phosphate acyltransferase |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| HO-1 | heme oxygenase |
| HPLC-ESI-MS | high-performance liquid chromatography/electrospray ionization tandem mass spectrometry |
| IBMX | 3-isobutyl-1-methylxanthine |
| Keap1 | kelch-like ECH-associated protein 1 |
| LDs | lipid droplets |
| MM | maintenance medium |
| MnSOD | manganese superoxide dismutase |
| MDI | differentiation medium |
| MPE | mango peel extract |
| MSE | mango seed extract |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| Nrf2 | NF-E2–related factor 2 |
| ORO | Oil Red O |
| PGC-1 | PPAR coactivator 1 |
| PPARα | peroxide proliferative activation receptor alfa |
| PPARγ | peroxide proliferative activation receptor gamma |
| ROS | Reactive oxygen species. |
| SREBP-1c | sterol regulatory element-binding protein-1c |
| SCD1 | stearoyl-CoA desaturase |
| TGs | triacylglycerols |
| WAT | white adipose tissue |
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The Epidemiology of Obesity. Metab. Clin. Exp. 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Conway, B.; Rene, A. Obesity as a Disease: No Lightweight Matter. Obes. Rev. 2004, 5, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engin, A.B. What Is Lipotoxicity? Adv. Exp. Med. Biol. 2017, 960, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef]
- Sletten, A.C.; Peterson, L.R.; Schaffer, J.E. Manifestations and Mechanisms of Myocardial Lipotoxicity in Obesity. J. Intern. Med. 2018, 284, 478–491. [Google Scholar] [CrossRef] [Green Version]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, Hypertension, and Cardiac Dysfunction. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Cardiomyopathy in Obesity, Insulin Resistance and Diabetes. J. Physiol. 2020, 598, 2977–2993. [Google Scholar] [CrossRef] [Green Version]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and Cancer Risk: Emerging Biological Mechanisms and Perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Berry, R.; Jeffery, E.; Rodeheffer, M.S. Weighing in on Adipocyte Precursors. Cell Metab. 2014, 19, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Park, J.; Choi, J.H. Revisiting PPARγ as a Target for the Treatment of Metabolic Disorders. BMB Rep. 2014, 47, 599–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habinowski, S.A.; Witters, L.A. The Effects of AICAR on Adipocyte Differentiation of 3T3-L1 Cells. Biochem. Biophys. Res. Commun. 2001, 286, 852–856. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.-O. Beneficial Effects of Natural Bioactive Compounds from Hibiscus Sabdariffa L. on Obesity. Molecules 2019, 24, 210. [Google Scholar] [CrossRef] [Green Version]
- Bu, S.; Yuan, C.Y.; Xue, Q.; Chen, Y.; Cao, F. Bilobalide Suppresses Adipogenesis in 3T3-L1 Adipocytes via the AMPK Signaling Pathway. Molecules 2019, 24, 3503. [Google Scholar] [CrossRef] [Green Version]
- Vinesh, D.; Neeru, V.; Sunil, S.; Ashok, K.; David, R. Lead Anti-Obesity Compounds from Nature. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1637–1653. [Google Scholar]
- De Blasio, A.; D’Anneo, A.; Lauricella, M.; Emanuele, S.; Giuliano, M.; Pratelli, G.; Calvaruso, G.; Carlisi, D. The Beneficial Effects of Essential Oils in Anti-Obesity Treatment. Int. J. Mol. Sci. 2021, 22, 11832. [Google Scholar] [CrossRef]
- Ansary, J.; Forbes-Hernández, T.Y.; Gil, E.; Cianciosi, D.; Zhang, J.; Elexpuru-Zabaleta, M.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Potential Health Benefit of Garlic Based on Human Intervention Studies: A Brief Overview. Antioxidants 2020, 9, 619. [Google Scholar] [CrossRef]
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D’Anneo, A. Multifaceted Health Benefits of Mangifera Indica L. (Mango): The Inestimable Value of Orchards Recently Planted in Sicilian Rural Areas. Nutrients 2017, 9, 525. [Google Scholar] [CrossRef]
- Mohan, C.; Deepak, M.; Viswanatha, G.; Savinay, G.; Hanumantharaju, V.; Rajendra, C.; Halemani, P.D. Anti-Oxidant and Anti-Inflammatory Activity of Leaf Extracts and Fractions of Mangifera Indica. Asian Pac. J. Trop. Med. 2013, 6, 311–314. [Google Scholar] [CrossRef]
- García-Rivera, D.; Delgado, R.; Bougarne, N.; Haegeman, G.; Vanden Berghe, W. Gallic Acid Indanone and Mangiferin Xanthone Are Strong Determinants of Immunosuppressive Anti-Tumour Effects of Mangifera Indica L. Bark in MDA-MB231 Breast Cancer Cells. Cancer Lett. 2011, 305, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Lauricella, M.; Lo Galbo, V.; Cernigliaro, C.; Maggio, A.; Palumbo Piccionello, A.; Calvaruso, G.; Carlisi, D.; Emanuele, S.; Giuliano, M.; D’Anneo, A. The Anti-Cancer Effect of Mangifera Indica L. Peel Extract Is Associated to ΓH2AX-Mediated Apoptosis in Colon Cancer Cells. Antioxidants 2019, 8, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sferrazzo, G.; Palmeri, R.; Vanella, L.; Parafati, L.; Ronsisvalle, S.; Biondi, A.; Basile, F.; Li Volti, G.; Barbagallo, I. Mangifera Indica L. Leaf Extract Induces Adiponectin and Regulates Adipogenesis. Int. J. Mol. Sci. 2019, 20, 3211. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Kim, H.; Barnes, R.C.; Talcott, S.T.; Mertens-Talcott, S.U. Obesity-Associated Diseases Biomarkers Are Differently Modulated in Lean and Obese Individuals and Inversely Correlated to Plasma Polyphenolic Metabolites After 6 Weeks of Mango (Mangifera indica L.) Consumption. Mol. Nutr. Food Res. 2018, 62, 1800129. [Google Scholar] [CrossRef]
- Lo Galbo, V.; Lauricella, M.; Giuliano, M.; Emanuele, S.; Carlisi, D.; Calvaruso, G.; De Blasio, A.; Di Liberto, D.; D’Anneo, A. Redox Imbalance and Mitochondrial Release of Apoptogenic Factors at the Forefront of the Antitumor Action of Mango Peel Extract. Molecules 2021, 26, 4328. [Google Scholar] [CrossRef]
- Morales, M.; Zapata, K.; Sagaste, C.A.; Angulo, A.A.; Rojano, B. Optimization of the Ultrasound-Assisted Extraction of Polyphenol, Mangiferin, and Its Antioxidant Expressionin Mango Peel (Mangifera Indica) Using Response Surface Methodology. Acta Sci. Pol. Technol. Aliment. 2020, 19, 5–14. [Google Scholar] [CrossRef]
- Taing, M.-W.; Pierson, J.-T.; Hoang, V.L.T.; Shaw, P.N.; Dietzgen, R.G.; Gidley, M.J.; Roberts-Thomson, S.J.; Monteith, G.R. Mango Fruit Peel and Flesh Extracts Affect Adipogenesis in 3T3-L1 Cells. Food Funct. 2012, 3, 828–836. [Google Scholar] [CrossRef]
- Taing, M.-W.; Pierson, J.-T.; Shaw, P.N.; Dietzgen, R.G.; Roberts-Thomson, S.J.; Gidley, M.J.; Monteith, G.R. Mango (Mangifera indica L.) Peel Extract Fractions from Different Cultivars Differentially Affect Lipid Accumulation in 3T3-L1 Adipocyte Cells. Food Funct. 2013, 4, 481–491. [Google Scholar] [CrossRef]
- Maggio, B.; Raimondi, M.V.; Raffa, D.; Plescia, F.; Scherrmann, M.-C.; Prosa, N.; Lauricella, M.; D’Anneo, A.; Daidone, G. Synthesis and Antiproliferative Activity of a Natural like Glycoconjugate Polycyclic Compound. Eur. J. Med. Chem. 2016, 122, 247–256. [Google Scholar] [CrossRef]
- Emanuele, S.; Notaro, A.; Palumbo Piccionello, A.; Maggio, A.; Lauricella, M.; D’Anneo, A.; Cernigliaro, C.; Calvaruso, G.; Giuliano, M. Sicilian Litchi Fruit Extracts Induce Autophagy versus Apoptosis Switch in Human Colon Cancer Cells. Nutrients 2018, 10, 1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grün, F.; Watanabe, H.; Zamanian, Z.; Maeda, L.; Arima, K.; Cubacha, R.; Gardiner, D.M.; Kanno, J.; Iguchi, T.; Blumberg, B. Endocrine-Disrupting Organotin Compounds Are Potent Inducers of Adipogenesis in Vertebrates. Mol. Endocrinol. 2006, 20, 2141–2155. [Google Scholar] [CrossRef] [PubMed]
- Celesia, A.; Morana, O.; Fiore, T.; Pellerito, C.; D’Anneo, A.; Lauricella, M.; Carlisi, D.; De Blasio, A.; Calvaruso, G.; Giuliano, M.; et al. ROS-Dependent ER Stress and Autophagy Mediate the Anti-Tumor Effects of Tributyltin (IV) Ferulate in Colon Cancer Cells. Int. J. Mol. Sci. 2020, 21, 8135. [Google Scholar] [CrossRef] [PubMed]
- Roh, C.; Jung, U. Screening of Crude Plant Extracts with Anti-Obesity Activity. Int. J. Mol. Sci. 2012, 13, 1710–1719. [Google Scholar] [CrossRef] [Green Version]
- Green, H.; Meuth, M. An Established Pre-Adipose Cell Line and Its Differentiation in Culture. Cell 1974, 3, 127–133. [Google Scholar] [CrossRef]
- Mota de Sá, P.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional Regulation of Adipogenesis. Compr Physiol. 2017, 7, 635–674. [Google Scholar] [CrossRef]
- Xiaoru, S.; Meiqi, W.; Xueqin, W.; Shuwen, D.; Na, F.; Qiang, P.; Yan, J.; Ling, Y.; Jiamin, X.; Yunfeng, L. Peroxisome Proliferator-Activated Receptor-γ: Master Regulator of Adipogenesis and Obesity. Curr. Stem Cell Res. Ther. 2016, 11, 282–289. [Google Scholar]
- Amri, E.; Bertrand, B.; Ailhaud, G.; Grimaldi, P. Regulation of Adipose Cell Differentiation. I. Fatty Acids Are Inducers of the AP2 Gene Expression. J. Lipid Res. 1991, 32, 1449–1456. [Google Scholar] [CrossRef]
- Prentice, K.J.; Saksi, J.; Hotamisligil, G.S. Adipokine FABP4 Integrates Energy Stores and Counterregulatory Metabolic Responses. J. Lipid Res. 2019, 60, 734–740. [Google Scholar] [CrossRef] [Green Version]
- Govers, R. Molecular Mechanisms of GLUT4 Regulation in Adipocytes. Diabetes Metab. 2014, 40, 400–410. [Google Scholar] [CrossRef]
- Song, N.-J.; Kim, S.; Jang, B.-H.; Chang, S.-H.; Yun, U.J.; Park, K.-M.; Waki, H.; Li, D.Y.; Tontonoz, P.; Park, K.W. Small Molecule-Induced Complement Factor D (Adipsin) Promotes Lipid Accumulation and Adipocyte Differentiation. PLoS ONE 2016, 11, e0162228. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the Complete Program of Cholesterol and Fatty Acid Synthesis in the Liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Tabor, D.E.; Kim, J.B.; Spiegelman, B.M.; Edwards, P.A. Transcriptional Activation of the Stearoyl-CoA Desaturase 2 Gene by Sterol Regulatory Element-Binding Protein/Adipocyte Determination and Differentiation Factor 1. J. Biol. Chem. 1998, 273, 22052–22058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magaña, M.M.; Lin, S.S.; Dooley, K.A.; Osborne, T.F. Sterol Regulation of Acetyl Coenzyme A Carboxylase Promoter Requires Two Interdependent Binding Sites for Sterol Regulatory Element Binding Proteins. J. Lipid Res. 1997, 38, 1630–1638. [Google Scholar] [CrossRef]
- Shimomura, I.; Shimano, H.; Korn, B.S.; Bashmakov, Y.; Horton, J.D. Nuclear Sterol Regulatory Element-Binding Proteins Activate Genes Responsible for the Entire Program of Unsaturated Fatty Acid Biosynthesis in Transgenic Mouse Liver. J. Biol. Chem. 1998, 273, 35299–35306. [Google Scholar] [CrossRef] [Green Version]
- Pyper, S.R.; Viswakarma, N.; Yu, S.; Reddy, J.K. PPARα: Energy Combustion, Hypolipidemia, Inflammation and Cancer. Nucl. Recept. Signal. 2010, 8, nrs.08002. [Google Scholar] [CrossRef] [Green Version]
- Haemmerle, G.; Moustafa, T.; Woelkart, G.; Büttner, S.; Schmidt, A.; van de Weijer, T.; Hesselink, M.; Jaeger, D.; Kienesberger, P.C.; Zierler, K.; et al. ATGL-Mediated Fat Catabolism Regulates Cardiac Mitochondrial Function via PPAR-α and PGC-1. Nat. Med. 2011, 17, 1076–1085. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-Activated Protein Kinase in Mechanism of Metformin Action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Cernigliaro, C.; D’Anneo, A.; Carlisi, D.; Giuliano, M.; Marino Gammazza, A.; Barone, R.; Longhitano, L.; Cappello, F.; Emanuele, S.; Distefano, A.; et al. Ethanol-Mediated Stress Promotes Autophagic Survival and Aggressiveness of Colon Cancer Cells via Activation of Nrf2/HO-1 Pathway. Cancers 2019, 11, 505. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Ajila, C.M.; Bhat, S.G.; Prasada Rao, U.J.S. Valuable Components of Raw and Ripe Peels from Two Indian Mango Varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.-J.; Ye, W.; Korivi, M. Nutritional Composition and Bioactive Compounds in Three Different Parts of Mango Fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Lorenzo, A.D.; Nabavi, S.F.; Talas, Z.S.; Nabavi, S.M. Polyphenols: Well Beyond The Antioxidant Capacity: Gallic Acid and Related Compounds as Neuroprotective Agents: You Are What You Eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef]
- Tanaka, M.; Sugama, A.; Sumi, K.; Shimizu, K.; Kishimoto, Y.; Kondo, K.; Iida, K. Gallic Acid Regulates Adipocyte Hypertrophy and Suppresses Inflammatory Gene Expression Induced by the Paracrine Interaction between Adipocytes and Macrophages in Vitro and in Vivo. Nutr. Res. 2020, 73, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Jeon, M.; Kim, Y.-S. Methyl Gallate, a Potent Antioxidant Inhibits Mouse and Human Adipocyte Differentiation and Oxidative Stress in Adipocytes through Impairment of Mitotic Clonal Expansion. BioFactors 2016, 42, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming Functional Fat: A Growing Understanding of Adipocyte Differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef]
- Rosen, E.D.; Sarraf, P.; Troy, A.E.; Bradwin, G.; Moore, K.; Milstone, D.S.; Spiegelman, B.M.; Mortensen, R.M. PPARγ Is Required for the Differentiation of Adipose Tissue In Vivo and In Vitro. Mol. Cell 1999, 4, 611–617. [Google Scholar] [CrossRef]
- Thompson, G.M.; Trainor, D.; Biswas, C.; LaCerte, C.; Berger, J.P.; Kelly, L.J. A High-Capacity Assay for PPARγ Ligand Regulation of Endogenous AP2 Expression in 3T3-L1 Cells. Anal. Biochem. 2004, 330, 21–28. [Google Scholar] [CrossRef]
- Osinski, V.; Bauknight, D.K.; Dasa, S.S.K.; Harms, M.J.; Kroon, T.; Marshall, M.A.; Garmey, J.C.; Nguyen, A.T.; Hartman, J.; Upadhye, A.; et al. In Vivo Liposomal Delivery of PPARα/γ Dual Agonist Tesaglitazar in a Model of Obesity Enriches Macrophage Targeting and Limits Liver and Kidney Drug Effects. Theranostics 2020, 10, 585–601. [Google Scholar] [CrossRef]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK Activators: Mechanisms of Action and Physiological Activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol Induces Brown-like Adipocyte Formation in White Fat through Activation of AMP-Activated Protein Kinase (AMPK) A1. Int. J. Obes. 2015, 39, 967–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Kang, R.; Bae, S.; Yoon, Y. AICAR, an Activator of AMPK, Inhibits Adipogenesis via the WNT/β-Catenin Pathway in 3T3-L1 Adipocytes. Int. J. Mol. Med. 2011, 28, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol Enhances Brown Adipocyte Formation and Function by Activating AMP-Activated Protein Kinase (AMPK) A1 in Mice Fed High-Fat Diet. Mol. Nutr. Food Res. 2017, 61, 1600746. [Google Scholar] [CrossRef] [Green Version]
- Han, M.H.; Kim, H.J.; Jeong, J.-W.; Park, C.; Kim, B.W.; Choi, Y.H. Inhibition of Adipocyte Differentiation by Anthocyanins Isolated from the Fruit of Vitis Coignetiae Pulliat Is Associated with the Activation of AMPK Signaling Pathway. Toxicol. Res. 2018, 34, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.-S.; Jeon, H.-J.; Lee, O.-H.; Lee, B.-Y. Dieckol, a Major Phlorotannin in Ecklonia Cava, Suppresses Lipid Accumulation in the Adipocytes of High-Fat Diet-Fed Zebrafish and Mice: Inhibition of Early Adipogenesis via Cell-Cycle Arrest and AMPKα Activation. Mol. Nutr. Food Res. 2015, 59, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single Phosphorylation Sites in Acc1 and Acc2 Regulate Lipid Homeostasis and the Insulin–Sensitizing Effects of Metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef] [Green Version]
- De Villiers, D.; Potgieter, M.; Ambele, M.A.; Adam, L.; Durandt, C.; Pepper, M.S. The Role of Reactive Oxygen Species in Adipogenic Differentiation. Adv. Exp. Med. Biol. 2018, 1083, 125–144. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J. Reactive Oxygen Species Facilitate Adipocyte Differentiation by Accelerating Mitotic Clonal Expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhang, Y.; Lu, W.; Liu, K. Mitochondrial Reactive Oxygen Species Regulate Adipocyte Differentiation of Mesenchymal Stem Cells in Hematopoietic Stress Induced by Arabinosylcytosine. PLoS ONE 2015, 10, e0120629. [Google Scholar] [CrossRef]
- Tormos, K.V.; Anso, E.; Hamanaka, R.B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chandel, N.S. Mitochondrial Complex III ROS Regulate Adipocyte Differentiation. Cell Metab. 2011, 14, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Emanuele, S.; Celesia, A.; D’Anneo, A.; Lauricella, M.; Carlisi, D.; De Blasio, A.; Giuliano, M. The Good and Bad of Nrf2: An Update in Cancer and New Perspectives in COVID-19. Int. J. Mol. Sci. 2021, 22, 7963. [Google Scholar] [CrossRef] [PubMed]
- Ooi, B.K.; Chan, K.-G.; Goh, B.H.; Yap, W.H. The Role of Natural Products in Targeting Cardiovascular Diseases via Nrf2 Pathway: Novel Molecular Mechanisms and Therapeutic Approaches. Front. Pharmacol. 2018, 9, 1308. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Jiang, Z.; Zhou, C.; Chen, K.; Li, X.; Wang, Z.; Wu, Z.; Ma, J.; Ma, Q.; Duan, W. Activation of Nrf2 by Sulforaphane Inhibits High Glucose-Induced Progression of Pancreatic Cancer via AMPK Dependent Signaling. Cell. Physiol. Biochem. 2018, 50, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, H.; Lu, C.; Shi, K.; Huang, H.; Zheng, Y.; Wang, Y.; Wang, D.; Wang, H.; Huang, W. AICAR, an AMP-Activated Protein Kinase Activator, Ameliorates Acute Pancreatitis-Associated Liver Injury Partially Through Nrf2-Mediated Antioxidant Effects and Inhibition of NLRP3 Inflammasome Activation. Front. Pharmacol. 2021, 12, 724514. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, L.V.; Savova, M.S.; Amirova, K.M.; Dinkova-Kostova, A.T.; Georgiev, M.I. Obesity and NRF2-Mediated Cytoprotection: Where Is the Missing Link? Pharmacol. Res. 2020, 156, 104760. [Google Scholar] [CrossRef]








| Compound | RT (min) | ESI [M − H] (m/z) | Molecular Formula | ppm | mg/100 g | ||
|---|---|---|---|---|---|---|---|
| Teor. | Exp. | ||||||
| 1 | Disaccaride | 1.5 | 341.1089 [M − H]− | 341.1089 [M − H]− | C12H22O11 | trace | trace |
| 2 | Quinic acid | 1.5 | 191.0561 [M − H]− | 191.0561 [M − H]− | C7H12O6 | trace | trace |
| 3 | Glucosyl gallate | 4.6 | 331.0671 [M − H]− | 331.06707 [M − H]− | C13H16O10 | 2.80 | 280 |
| 4 | Gallic acid | 5.8 | 169.0142 [M − H]− | 169.01425 [M − H]− | C7H6O5 | 1.3 | 130 |
| 5 | Methylgallate | 15.3 | 183.0299 [M − H]− | 183.02990 [M − H]− | C8H8O5 | 25.2 | 2520 |
| 6 | Mangiferin | 20.9 | 421.0776 [M − H]− | 421.07763 [M − H]− | C19H18O11 | 0.5 | 50 |
| 7 | Methyl-digallate ester isomer | 22.6 | 335.04086 [M-H]- | 12.1 | 1210 | ||
| 8 | Maclurin tri-O-galloyl-glucoside | 29.0 | 879.1262 [M − H]− | 879.11651 [M − H]− | C40H32O23 | trace | trace |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratelli, G.; Carlisi, D.; D’Anneo, A.; Maggio, A.; Emanuele, S.; Palumbo Piccionello, A.; Giuliano, M.; De Blasio, A.; Calvaruso, G.; Lauricella, M. Bio-Waste Products of Mangifera indica L. Reduce Adipogenesis and Exert Antioxidant Effects on 3T3-L1 Cells. Antioxidants 2022, 11, 363. https://doi.org/10.3390/antiox11020363
Pratelli G, Carlisi D, D’Anneo A, Maggio A, Emanuele S, Palumbo Piccionello A, Giuliano M, De Blasio A, Calvaruso G, Lauricella M. Bio-Waste Products of Mangifera indica L. Reduce Adipogenesis and Exert Antioxidant Effects on 3T3-L1 Cells. Antioxidants. 2022; 11(2):363. https://doi.org/10.3390/antiox11020363
Chicago/Turabian StylePratelli, Giovanni, Daniela Carlisi, Antonella D’Anneo, Antonella Maggio, Sonia Emanuele, Antonio Palumbo Piccionello, Michela Giuliano, Anna De Blasio, Giuseppe Calvaruso, and Marianna Lauricella. 2022. "Bio-Waste Products of Mangifera indica L. Reduce Adipogenesis and Exert Antioxidant Effects on 3T3-L1 Cells" Antioxidants 11, no. 2: 363. https://doi.org/10.3390/antiox11020363