Effect of Magnesium Ion on the Radical-Scavenging Rate of Pterostilbene in an Aprotic Medium: Mechanistic Insight into the Antioxidative Reaction of Pterostilbene
Abstract
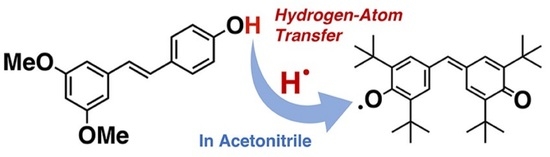
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spectral and Kinetic Measurements
2.3. Theoretical Calculations
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health benefits and molecular mechanisms of resveratrol: A narrative review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, Y.; Ho, C.; Pan, M. Recent advances in health benefits of stilbenoids. J. Agric. Food Chem. 2021, 69, 10036–10057. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seo, H.; Yokoyama, W. Chemistry of pterostilbene and its metabolic effects. J. Agric. Food Chem. 2020, 68, 12836–12841. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, X.; Xu, L.; Liu, D.; Di, S.; Li, W.; Zhang, J.; Zhang, H.; Li, X.; Han, J.; et al. Pterostilbene: Mechanisms of its action as oncostatic agent in cell models and in vivo studies. Pharmacol. Res. 2019, 145, 104265. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.H.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [Green Version]
- McCormack, D.; McFadden, D. A review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell. Longev. 2013, 2013, 575482. [Google Scholar] [CrossRef] [Green Version]
- Pandithavidana, D.R.; Jayawardana, S.B. Comparative study of antioxidant potential of selected dietary vitamins; computational insights. Molecules 2019, 24, 1646. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, I.; Shimada, T.; Ohkuob, K.; Manda, S.; Shimizu, T.; Urano, S.; Okuda, H.; Miyata, N.; Ozawa, T.; Anzai, K.; et al. Involvement of electron transfer in the radical-scavenging reaction of resveratrol. Chem. Lett. 2007, 36, 1276–1277. [Google Scholar] [CrossRef]
- Nakanishi, I.; Kawashima, T.; Ohkubo, K.; Kanazawa, H.; Inami, K.; Mochizuki, M.; Fukuahra, K.; Okuda, H.; Ozawa, T.; Itoh, S.; et al. Electron-transfer mechanism in radical-scavenging reactions by a vitamin E model in a protic medium. Org. Biomol. Chem. 2005, 3, 626–629. [Google Scholar] [CrossRef]
- Nakanishi, I.; Ohkubo, K.; Miyazaki, K.; Hakamata, W.; Urano, S.; Ozawa, T.; Okuda, H.; Fukuzumi, S.; Ikota, N.; Fukuhara, K. A planar catechin analogue having a more negative oxidation potential than (+)-catechin as an electron-transfer antioxidant against a peroxyl radical. Chem. Res. Toxicol. 2004, 17, 26–31. [Google Scholar] [CrossRef]
- Nakanishi, I.; Uto, Y.; Ohkubo, K.; Miyazaki, K.; Yakumaru, H.; Urano, S.; Okuda, H.; Ueda, J.; Ozawa, T.; Fukuhara, K.; et al. Efficient radical scavenging ability of artepillin C, a major component of Brazilian propolis, and the mechanism. Org. Biomol. Chem. 2003, 1, 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, I.; Miyazaki, K.; Shimada, T.; Ohkubo, K.; Urano, S.; Ikota, N.; Ozawa, T.; Fukuzumi, S.; Fukuhara, K. Effects of metal ions distinguishing between one-step hydrogen- and electron-transfer mechanisms for the radical-scavenging reaction of (+)-catechin. J. Phys. Chem. A 2002, 106, 11123–11126. [Google Scholar] [CrossRef]
- Nakanishi, I.; Fukuhara, K.; Shimada, T.; Ohkubo, K.; Iizuka, Y.; Inami, K.; Mochizuki, M.; Urano, S.; Itoh, S.; Miyata, N.; et al. Effects of magnesium ion on kinetic stability and spin distribution of phenoxyl radical derived from a vitamin E analogue; mechanistic insight into antioxidative hydrogen transfer reaction of vitamin E. J. Chem. Soc. Perkin Trans. 2 2002, 1520–1524. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K. Metal ion-coupled and decoupled electron transfer. Coord. Chem. Rev. 2010, 254, 372–385. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K.; Morimoto, Y. Mechanism of metal ion-coupled electron transfer. Phys. Chem. Chem. Phys. 2012, 14, 8472–8484. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Nakanishi, I.; Ohkubo, K.; Ohno, A.; Mizuno, M.; Fukuzumi, S.; Matsumoto, K.; Fukuhara, K. Synthesis and radical-scavenging activity of C-methylated fisetin analogues. Bioorg. Med. Chem. 2019, 27, 1720–1727. [Google Scholar] [CrossRef]
- Sekine-Suzuki, E.; Nakanishi, I.; Imai, K.; Ueno, M.; Shimokawa, T.; Matsumoto, K.; Fukuhara, K. Efficient protective activity of a planar catechin analogue against radiation-induced apoptosis in rat thymocytes. RSC Adv. 2018, 8, 10158–10162. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, M.; Nakanishi, I.; Matsumoto, K.; Fukuhara, K. Enhanced radical scavenging activity of a procyanidin B3 analogue comprised of a dimer of planar catechin. Bioorg. Med. Chem. Lett. 2017, 27, 5010–5013. [Google Scholar] [CrossRef]
- Imai, K.; Nakanishi, I.; Ohkubo, K.; Ohba, Y.; Arai, T.; Mizuno, M.; Fukuzumi, S.; Matsumoto, K.; Fukuhara, K. Synthesis of methylated quercetin analogues for enhancement of radical-scavenging activity. RSC Adv. 2017, 7, 17968–17979. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, M.; Nakanishi, I.; Matsubayashi, S.; Imai, K.; Arai, T.; Matsumoto, K.; Fukuhara, K. Synthesis and antioxidant activity of a procyanidin B3 analogue. Bioorg. Med. Chem. Lett. 2017, 27, 1041–1044. [Google Scholar] [CrossRef]
- Uzura, S.; Sekine-Suzuki, E.; Nakanishi, I.; Sonoda, M.; Tanimori, S. A facile and rapid access to resveratrol derivatives and their radioprotective activity. Bioorg. Med. Chem. Lett. 2016, 26, 3886–3891. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Nakanishi, I.; Ohno, A.; Kurihara, M.; Miyata, N.; Matsumoto, K.; Nakamura, A.; Fukuhara, K. Synthesis and radical-scavenging activity of a dimethylcatechin analogue. Bioorg. Med. Chem. Lett. 2014, 24, 2582–2584. [Google Scholar] [CrossRef] [PubMed]
- Inami, K.; Nakanishi, I.; Morita, M.; Furukawa, M.; Ohkubo, K.; Fukuzumi, S.; Mochizuki, M. The high stability of intermediate radicals enhances the radical-scavenging activity of aminochromanols. RSC Adv. 2012, 2, 12714–12717. [Google Scholar] [CrossRef]
- Kawashima, T.; Manda, S.; Uto, Y.; Ohkubo, K.; Hori, H.; Matsumoto, K.; Fukuhara, K.; Ikota, N.; Fukuzumi, S.; Ozawa, T.; et al. Kinetic and mechanism for the scavenging reaction of the 2,2-diphenyl-1-picrylhydrazyl radical by synthetic artepillin C analogues. Bull. Chem. Soc. Jpn. 2012, 85, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Imai, K.; Nakanishi, I.; Anzai, K.; Ozawa, T.; Miyata, N.; Urano, S.; Okuda, H.; Nakamura, A.; Fukuhara, K. Synthesis and enhanced radical scavenging activity of a conformationally constrained epigallocatechin analogue. Chem. Lett. 2011, 40, 1417–1419. [Google Scholar] [CrossRef]
- Fukuhara, K.; Ohno, A.; Nakanishi, I.; Imai, K.; Nakamura, A.; Anzai, K.; Miyata, N.; Okuda, H. Novel ninhydrin adduct of catechin with potent antioxidantive activity. Tetrahedron Lett. 2009, 50, 6989–6992. [Google Scholar] [CrossRef]
- Fukuhara, K.; Nakanishi, I.; Ohkubo, K.; Obara, Y.; Tada, A.; Imai, K.; Ohno, A.; Nakamura, A.; Ozawa, T.; Urano, S.; et al. Intramolecular base-accelerated radical-scavenging reaction of a planar catechin derivative bearing a lysine moiety. Chem. Commun. 2009, 6180–6182. [Google Scholar] [CrossRef]
- Fukuhara, K.; Nakanishi, I.; Matsuoka, A.; Matsumura, T.; Honda, S.; Hayashi, M.; Ozawa, T.; Miyata, N.; Saito, S.; Ikota, N.; et al. Effect of methyl substitution on antioxidantive property and genotoxicity of resveratrol. Chem. Res. Toxicol. 2008, 21, 282–287. [Google Scholar] [CrossRef]
- Manda, S.; Nakanishi, I.; Ohkubo, K.; Uto, Y.; Kawashima, T.; Fukuhara, K.; Okuda, H.; Hori, H.; Ozawa, T.; Ikota, N.; et al. Enhanced radical-scavenging activity of naturally-oriented artepillin C derivatives. Chem. Commun. 2008, 626–628. [Google Scholar] [CrossRef]
- Hakamata, W.; Nakanishi, I.; Masuda, Y.; Shimizu, T.; Higuchi, H.; Nakamura, Y.; Saito, S.; Urano, S.; Oku, T.; Ozawa, T.; et al. Planar catechin analogue with alkyl side chains, a potent antioxidant and an α-glucosidase inhibitor. J. Am. Chem. Soc. 2006, 128, 6524–6525. [Google Scholar] [CrossRef]
- Fukuhara, K.; Nakanishi, I.; Kansui, H.; Sugiyama, E.; Kimura, M.; Shimada, T.; Urano, S.; Yamaguchi, K.; Miyata, N. Enhanced radical-scavenging activity of a planar catechin analogue. J. Am. Chem. Soc. 2002, 124, 5952–5953. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Noguchi, N.; Niki, E. Galvinoxyl method for standardizing electron and proton donation activity. Methods Enzymol. 2001, 335, 157–166. [Google Scholar] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Mann, C.K.; Barnes, K.K. Electrochemical Reactions in Nonaqueous Systems; Mercel Dekker: New York, NY, USA, 1970. [Google Scholar]
- Fukuzumi, S.; Lee, Y.; Nam, W. Deuterium kinetic isotope effects as redox mechanistic criterions. Bull. Korean Chem. Soc. 2021, 42, 1558–1568. [Google Scholar] [CrossRef]






| Compound | kH/M−1 s−1 | DHT/ kcal mol−1 | IP/kcal mol−1 | EHOMO/kcal mol−1 | Epa/V vs. SCE |
|---|---|---|---|---|---|
| RSV | 4.1 | 398.4 | 133.3 | –157.7 | +1.10 |
| PTS | 1.3 × 10 | 398.2 | 132.6 | –158.9 | +1.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, I.; Shoji, Y.; Ohkubo, K.; Ueno, M.; Shimoda, K.; Matsumoto, K.-i.; Fukuhara, K.; Hamada, H. Effect of Magnesium Ion on the Radical-Scavenging Rate of Pterostilbene in an Aprotic Medium: Mechanistic Insight into the Antioxidative Reaction of Pterostilbene. Antioxidants 2022, 11, 340. https://doi.org/10.3390/antiox11020340
Nakanishi I, Shoji Y, Ohkubo K, Ueno M, Shimoda K, Matsumoto K-i, Fukuhara K, Hamada H. Effect of Magnesium Ion on the Radical-Scavenging Rate of Pterostilbene in an Aprotic Medium: Mechanistic Insight into the Antioxidative Reaction of Pterostilbene. Antioxidants. 2022; 11(2):340. https://doi.org/10.3390/antiox11020340
Chicago/Turabian StyleNakanishi, Ikuo, Yoshimi Shoji, Kei Ohkubo, Megumi Ueno, Kei Shimoda, Ken-ichiro Matsumoto, Kiyoshi Fukuhara, and Hiroki Hamada. 2022. "Effect of Magnesium Ion on the Radical-Scavenging Rate of Pterostilbene in an Aprotic Medium: Mechanistic Insight into the Antioxidative Reaction of Pterostilbene" Antioxidants 11, no. 2: 340. https://doi.org/10.3390/antiox11020340