Does the Type Matter? Verification of Different Tea Types’ Potential in the Synthesis of SeNPs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Tea Samples and Infusion Preparation
2.3. Chromatographic Analysis of Polyphenols in Tea Extracts
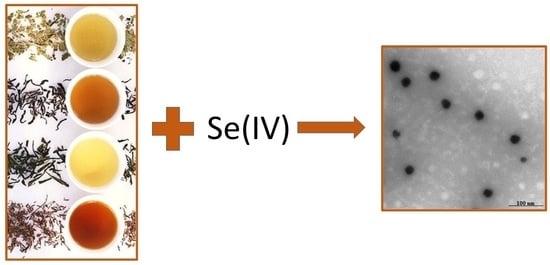
2.4. Synthesis and Characterization of Selenium Nanoparticles
2.5. Antioxidant Activity Measurements
2.6. Statistical Analysis
3. Results
3.1. Characterization of Teas Used for SeNP Synthesis
3.2. Synthesis of SeNPs
3.3. Characterization of SeNPs
4. Discussion
4.1. Polyphenolic Content and Antioxidant Activity of Tea Extracts
4.2. Synthesis of SeNPs
4.3. Characterization of SeNPs in Terms of Size and Morphology
4.4. Antioxidant Activity of Synthesized SeNPs
4.5. Comparison of Properties of SeNPs Obtained Using Tea Extracts and by Chemical Synthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chupeerach, C.; Aursalung, A.; Watcharachaisoponsiri, T.; Whanmek, K.; Thiyajai, P.; Yosphan, K.; Sritalahareuthai, V.; Sahasakul, Y.; Santivarangkna, C.; Suttisansanee, U. The effect of steaming and fermentation on nutritive values, antioxidant activities, and inhibitory properties of tea leaves. Foods 2021, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.J.; Wei, X.L.; Liu, H.Y.; Li, H.; Xia, Y.; Wu, D.T.; Zhang, P.Z.; Gandhi, G.R.; Li, H.B.; Gan, R.Y. State-of-the-art review of dark tea: From chemistry to health benefits. Trends Food Sci. Techol. 2021, 109, 126–138. [Google Scholar] [CrossRef]
- Klepacka, J.; Tońska, E.; Rafałowski, R.; Czarnowska-Kujawska, M.; Opara, B. Tea as a source of biologically active compounds in the human diet. Molecules 2021, 26, 1487. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; BiBi, J.; Kamboh, A.A.; Suheryani, I.; Kakar, I.; Fazlani, S.A.; FangFang, X.; Yunjuan, L.; Kakar, M.U.; Abd El-Hack, M.E.; et al. Pharcacological values and therapheutic properties of black tea (Camelia sinensis): A comprehensive overview. Biomed. Pharmacoter. 2018, 100, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zheng, X.; Yang, Y.; Bu, P. The Effect of Black Tea Supplementation on Blood Pressure: A systematic review and dose–response meta-analysis of randomized controlled trials. Food Funct. 2021, 12, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.K.; Inoue, M. Green tea and cancer and cardiometabolic diseases: A review of the current epidemiological evidence. Eur. J. Clin. Nutr. 2021, 75, 865–876. [Google Scholar] [CrossRef]
- Liu, Z.; Vincken, J.-P.; de Bruijn, W.J. Tea phenolics as prebiotics. Trends Food Sci. Tech. 2022, 127, 156–168. [Google Scholar] [CrossRef]
- Xie, G.; Yan, J.; Lu, A.; Kun, J.; Wang, B.; Song, C.; Tong, H.; Meng, Q. Characterizing relationship between chemicals and in vitro bioactivities of teas made by six typical processing methods using a single Camellia sinensis cultivar, Meizhan. Bioengineered 2021, 12, 1251–1263. [Google Scholar] [CrossRef]
- Zhao, C.N.; Tang, G.Y.; Cao, S.Y.; Xu, R.Y.; Liu, Q.; Mao, Q.Q.; Shang, A.; Li, H.B. Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Applications of tea (Camellia sinensis) and its active constituents in cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef]
- Dai, J.; Sameen, D.E.; Zeng, Y.; Li, S.; Qin, W.; Liu, Y. An overview of tea polyphenols as bioactive agents for food packaging applications. LWT-Food Sci. Technol. 2022, 167, 113845. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; He, J.; Hong, Z.; Tan, R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosa microspheres. Inter. J. Nanomed. 2017, 12, 4527–4539. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidokova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, K.S. Role of nano-selenium in health and enviromnemt. J. Biotechnol. 2021, 325, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, N.; Usjak, D.; Milenkovic, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanovic, M.M. Comparative study of the antimicrobial activity of selenium nanoparticles with different surface chemistry and structure. Front. Bioeng. Biotechnol. 2021, 8, 1591. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzyńska, K. The influence of synthesis conditions on the antioxidant activity of selenium nanoparticles. Molecules 2022, 27, 2486. [Google Scholar] [CrossRef]
- Gu, X.; Gao, C. New horizons for selenium in animal nutrition and functional foods. Anim. Nutr. 2022, 11, 80–86. [Google Scholar] [CrossRef]
- Tzeng, W.Y.; Tseng, Y.H.; Yeh, T.T.; Tu, C.M.; Sankar, R.; Chen, Y.H.; Huang, B.H.; Chou, F.C.; Luo, C.W. Selenium nanoparticles prepared by femtosecond laser-induced plasma shock wave. Opt. Express 2020, 28, 685–694. [Google Scholar] [CrossRef]
- Qin, J.; Qiu, G.; Jian, J.; Zhou, H.; Yang, L.; Charnas, A.; Zemlyanov, D.Y.; Xu, C.Y.; Xu, X.; Wu, W. Controlled growth of large-size 2D selenium nanosheet and its electronic and optoelectronic applications. ACS Nano 2017, 11, 10222–10229. [Google Scholar] [CrossRef]
- Shar, A.H.; Lakhan, M.N.; Wang, J.; Ahmed, M.; Alali, K.T.; Ahmed, R.; Ali, I.; Dayo, A.Q. Facile synthesis and characterization of selenium nanoparticles by the hydrothermal approach. Dig. J. Nanomater. Biostruct. 2019, 14, 867–872. [Google Scholar]
- Panahi-Kalamuei, M.; Mousavi-Kamazani, M.; Salavati-Niasari, M.; Hosseinpour-Mashkani, S.M. A simple sonochemical approach for synthesis of selenium nanostructures and investigation of its light harvesting application. Ultrason. Sonochem. 2016, 23, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Bartosiak, M.; Giersz, J.; Jankowski, K. Analytical monitoring of selenium nanoparticles green synthesis using photochemical vapor generation coupled with MIP-OES and UV–Vis spectrophotometry. Microchem. J. 2019, 145, 1169–1175. [Google Scholar] [CrossRef]
- Chen, N.; Yao, P.; Zhang, W.; Zhang, Y.; Xin, N.; Wei, H.; Zhang, T.; Zhao, C. Selenium nanoparticles: Enhanced nutrition and beyond. Crit. Rev. Food Sci. Nutr. 2022, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K.; Sentkowska, A. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostruct. Chem. 2022, 12, 467–480. [Google Scholar] [CrossRef]
- Shi, X.D.; Tian, Y.Q.; Wu, J.L.; Wang, S.Y. Synthesis, characterization, and biological activity of selenium nanoparticles conjugated with polysaccharides. Crit. Rev. Food Sci. Nutr. 2021, 61, 2225–2236. [Google Scholar] [CrossRef]
- Korde, P.; Ghotekar, S.; Pagar, T.; Pansambal, S.; Oza, R.; Mane, D. Plant extract assisted eco-benevolent synthesis of selenium nanoparticles- a review on plant parts involved, characterization and their recent applications. J. Chem. Rev. 2020, 2, 157–168. [Google Scholar]
- Sentkowska, A. The potential of traditionally used medicinal plants for the synthesis of selenium nanoparticles. Nat. Prod. Res. 2022, 1–5. [Google Scholar] [CrossRef]
- Xin, H.; Yang, X.; Liu, X.; Tang, X.; Weng, L.; Han, Y. Biosynthesis of iron nanoparticles using Tie Guanyin tea extract for degradation of bromothymol blue. J. Nanotechnol. 2016, 2016, 4059591. [Google Scholar] [CrossRef]
- Huang, L.; Wenga, X.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron-based nanoparticles using oolong tea extract for the degradation of malachite green. Spectrochim. Acta Part A 2014, 117, 801–804. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008, 10, 859–862. [Google Scholar] [CrossRef]
- Elbossaty, W.F. Green tea as biological system for the synthesis of silver nanoparticles. J. Biotechnol. Biomater. 2017, 7, 31000269. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea and healt: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Stangl, V.; Lorenz, M.; Stangl, K. The role of tea and tea flavonoids in cardiovascular health. Mol. Nutr. Food Res. 2006, 50, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Jenny, T.; Mao, M.D. White Tea: The Plants, Processing, Manufacturing, and Potential Health Benefits. In Tea in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 3, pp. 33–40. [Google Scholar]
- Sentkowska, A.; Biesaga, M.; Pyrzynska, K. Effects of operation parameters on HILIC separation of flavonoids on zwitterionic column. Talanta 2013, 115, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1987, 28, 1057–1060. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E.; Erçag, E. The cupric reducing antioxidant capacity and polyphenolic content of some herbal teas. Inter. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Palacios-Morillo, A.; Alcazar, A.; de Pablos, F.; Jurando, J.M. Differentiation of tea varieties using UV-Vis spectra and pattern recognition techniques. Spectrochim. Acta Part A Mol. Biomol. Spec. 2013, 103, 79–83. [Google Scholar] [CrossRef]
- Fernandez, P.L.; Martin, M.J.; Gonzalez, A.G.; Pablos, F. HPLC determination of catechins and caffeine in tea. Differentiation of green, black and instant teas. Analyst 2000, 125, 421–425. [Google Scholar] [CrossRef]
- Roberts, E.A.H.; Smith, R.F. Spectrophotometric measurements of theaflavins and thearubigins in black tea liquors in assessments of quality in teas. Analyst 1961, 20, 141–151. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of Antioxidant Potency of Commonly Consumed Polyphenol-Rich Beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- Liu, Z.; Epicier, T.; Lefkir, Y.; Vitrant, G.; Destouches, N. HAADF-STEM characterization and simulation of nanoparticle distributions in an inhomogeneous matrix. J. Micros. 2017, 266, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Dróżdż, P.; Biesaga, M.; Pyrzynska, K. Evaluation of the antioxidant properties of fruit and flavoured black teas. Eur. J. Nutr. 2011, 50, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Sing, A.K.; Seth, P.; Anthony, P.; Husain, M.M.; Madhavan, S.; Mukhtar, H.; Maheshwari, R.K. Green tea constituent epigallocatechin-3-gallate inhibits angiogenic differentiation of human endothelial cells. Arch. Biochem. Biophys. 2002, 401, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Dwyer, J.; Bhagwat, S.; Hayowitz, D.; Holden, J.; Eldridge, A.L. Major flavonoids in dry tea. J. Food Comp. Anal. 2005, 18, 487–501. [Google Scholar] [CrossRef]
- Castro-Alves, V.C.; Cordenunsi, B.R. Total soluble phenolic compounds quantification is not as simple as it seems. Food Anal Meth. 2015, 8, 873–884. [Google Scholar] [CrossRef]
- Lin, Z.H.; Chris-Wang, C.R. Evidence on the size-dependent absorption spectral evolution of selenium nanoparticles. Mater. Chem. Phys. 2005, 92, 591–594. [Google Scholar] [CrossRef]
- Hughes, J.M.; Budd, P.M.; Grieve, A.; Dutta, P.; Tiede, K.; Lewis, J. Highly monodisperse, lanthanide-containing polystyrene nanoparticles as potential standard reference materials for environmental “nano” fate analysis. J. Appl. Polym. Sci. 2015, 132, 42069. [Google Scholar] [CrossRef]
- Galić, E.; Radić, K.; Golub, N.; Cepo, D.V.; Kalcec, N.; Vrcek, E.; Vinkovic, T. Utilization of olive pomace in green synthesis of selenium nanoparticles: Physico-chemical Ccharacterization, bioaccessibility and biocompatibility. Inter. J. Molec. Sci. 2022, 23, 9128. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Z.; Niu, S.; He, Y.; Wang, G.; Pei, X.; Tao, W.; Wang, M. Preparation, characteristics and feeble induced-apoptosis performance of non-dialysis requiring selenium nanoparticles@chitosan. Mater. Des. 2019, 182, 108024. [Google Scholar] [CrossRef]
- Guisbiers, G.; Wang, Q.; Khachatryan, E.; Mimun, L.C.; Mendoza-Cruz, R.; Larese-Casanova, P.; Webster, T.J.; Nash, K.I. Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int. J. Nanomed. 2016, 11, 3731–3736. [Google Scholar] [CrossRef]



| Black Tea | Green Tea | Red Tea | White Tea | |
|---|---|---|---|---|
| Flavonoids | ||||
| Catechin | 4.76 ± 0.201 a | 11.7 ± 0.352 b | 6.21 ± 0.019 c | 20.3 ± 0.521 d |
| Epicatechin | 0.296 ± 0.010 a | 0.231 ± 0.007 b | 0.252 ± 0.010 c | 0.360 ± 0.014 d |
| EGCG | 30.1 ± 0.100 a | 70.0 ± 0.201 b | 6.92 ± 0.020 c | 64.0 ± 0.190 d |
| Rutin | 25.8 ± 0.772 a | 5.72 ± 0.171 b | 12.5 ± 0.375 c | 18.6 ± 0.56 d |
| Polyphenolic Acids | ||||
| pHBA | 38.3 ± 1.53 a | 7.92 ± 0.158 b | 17.8 ± 0.535 c | 13.5 ± 0.540 d |
| Gallic acid | 3.74 ± 0.140 a | 0.950 ± 0.030 b | 6.36 ± 0.190 c | 1.81 ± 0.054 d |
| Chlorogenic acid | 2.23 ± 0.090 a | 0.721 ± 0.022 b | 0.637 ± 0.019 c | 1.47 ± 0.044 d |
| p-coumaric acid | 4.47 ± 0.130 a | 1.72 ± 0.052 b | 7.16 ± 0.280 c | 4.85 ± 0.145 d |
| Protocatechuic acid | 0.378 ± 0.007 a | 0.177 ± 0.001 b | 0.300 ± 0.020 c | 0.224 ± 0.011 d |
| Caffeic acid | 0.103 ± 0.003 a | <LOD 2 | 0.535 ± 0.010 b | 0.090 ± 0.002 a |
| Antioxidant Capacity * | |||||
|---|---|---|---|---|---|
| FC [mg GA/L] | CUPRAC [mmol TrE/L] | DPPH [mmol TrE/L] | OH [%] | AOX Index | |
| Tea Infusions | |||||
| Black tea | 796.1 ± 2.25 a | 19.44 ± 0.70 a | 13.00 ± 0.30 a | 18.20 ± 0.07 a | 94.8 ± 2.3 a |
| Green tea | 1196 ± 3.78 b | 19.73 ± 0.60 a | 17.71 ± 0.40 b | 30.43 ± 0.92 b | 96.3 ± 2.8 b |
| Red tea | 848.7 ± 2.13 c | 14.70 ± 0.50 b | 12.31 ± 0.30 c | 24.25 ± 0.80 c | 97.8 ± 2.7 c |
| White tea | 1414 ± 4.21 d | 24.62 ± 0.91 c | 17.36 ± 0.51 d | 26.15 ± 0.79 d | 97.4 ± 3.1 c |
| Selenium Nanoparticles | |||||
| BSeNPs | 350.8 ± 1.61 e | 8.951 ± 0.27 d | 5.42 ± 0.19 e | 98.88 ± 1.2 e | 97.4 ± 2.5 c |
| GSeNPs | 176.9 ± 0.50 f | 9.080 ± 0.30 d | 5.33 ± 0.11 e | 97.42 ± 1.3 e | 97.1 ± 1.9 c |
| RSeNPs | 240.1 ± 0.87 g | 7.830 ± 0.30 e | 4.52 ± 0.17 f | 78.41 ± 1.5 f | 94.2 ± 2.1 a |
| WSeNPs | 598.8 ± 2.25 h | 10.33 ± 0.41 f | 6.72 ± 0.20 g | 80.43 ± 1.6 g | 97.3 ± 2.4 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sentkowska, A.; Pyrzynska, K. Does the Type Matter? Verification of Different Tea Types’ Potential in the Synthesis of SeNPs. Antioxidants 2022, 11, 2489. https://doi.org/10.3390/antiox11122489
Sentkowska A, Pyrzynska K. Does the Type Matter? Verification of Different Tea Types’ Potential in the Synthesis of SeNPs. Antioxidants. 2022; 11(12):2489. https://doi.org/10.3390/antiox11122489
Chicago/Turabian StyleSentkowska, Aleksandra, and Krystyna Pyrzynska. 2022. "Does the Type Matter? Verification of Different Tea Types’ Potential in the Synthesis of SeNPs" Antioxidants 11, no. 12: 2489. https://doi.org/10.3390/antiox11122489