Folic Acid Improves Parkin-Null Drosophila Phenotypes and Transiently Reduces Vulnerable Dopaminergic Neuron Mitochondrial Hydrogen Peroxide Levels and Glutathione Redox Equilibrium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drosophila Maintenance, Folic Acid Administration, and Genotypes
2.2. Survival Assay
2.3. Negative Geotaxis Assay
2.4. Measuring Mitochondrial Hydrogen Peroxide Levels and Glutathione Redox Equilibrium in Dopaminergic Neurons
2.4.1. Drosophila Brain Dissection and Immunofluorescence
2.4.2. Image Capture and Analysis
2.5. Liquid Chromatography Tandem Mass Spectrometry Analysis of Small Molecular Weight Thiols/Disulfides
2.5.1. Preparation of Drosophila Head Lysates
2.5.2. Chemicals and Reagents
2.5.3. Sample Preparation for LC-MS Analysis
2.5.4. Liquid Chromatography Tandem Mass Spectrometry
2.5.5. Protein Assay
2.5.6. Statistical Analyses
3. Results
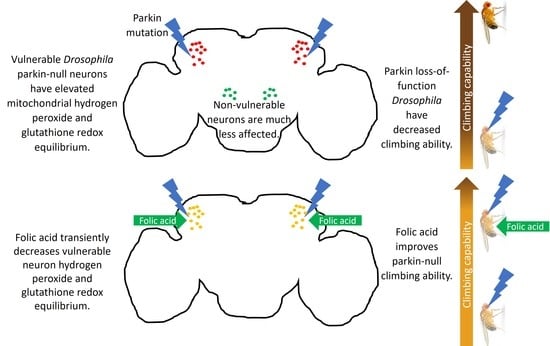
3.1. Decreases in Parkin-Null Mutant Climbing and Median Survival Accompany Increased Hydrogen Peroxide Levels and Glutathione Redox Equilibrium in Vulnerable Dopaminergic Neuron Mitochondria
3.2. Hydrogen Peroxide Levels Are Only Transiently Elevated, and Glutathione Redox Equilibrium Is Unaffected in Non-Vulnerable PPM3 Mitochondria
3.3. Folic Acid Administration Improves Climbing Behavior and Increases Lifespan in Parkin-Null Drosophila
3.4. Folic Acid Administration Transiently Decreases Hydrogen Peroxide and Glutathione Redox Equilibrium in Parkin-Null PPL1 Mitochondria
3.5. Folic Acid Supplementation Does Not Address Oxidative Stress in Heads of Parkin-Null Flies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buhlman, L.M. Parkin loss-of-function pathology: Premature neuronal senescence induced by high levels of reactive oxygen species? Mech. Ageing Dev. 2016, 161, 112–120. [Google Scholar] [CrossRef]
- Whitworth, A.J.; Theodore, D.A.; Greene, J.C.; Beneš, H.; Wes, P.D.; Pallanck, L.J. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 8024. [Google Scholar] [CrossRef] [Green Version]
- Strausfeld, N.J.; Hirth, F. Deep Homology of Arthropod Central Complex and Vertebrate Basal Ganglia. Science 2013, 340, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Bose, A.; Beal, M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016, 139, 216–231. [Google Scholar] [CrossRef]
- Bernardini, J.P.; Brouwer, J.M.; Tan, I.K.; Sandow, J.J.; Huang, S.; Stafford, C.A.; Bankovacki, A.; Riffkin, C.D.; Wardak, A.Z.; Czabotar, P.E.; et al. Parkin inhibits BAK and BAX apoptotic function by distinct mechanisms during mitophagy. Embo J. 2019, 38, e99916. [Google Scholar] [CrossRef]
- Narendra, D.; Tanaka, A.; Suen, D.-F.; Youle, R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008, 183, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Vincow, E.S.; Merrihew, G.; Thomas, R.E.; Shulman, N.J.; Beyer, R.P.; MacCoss, M.J.; Pallanck, L.J. The PINK1–Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 6400–6405. [Google Scholar] [CrossRef] [Green Version]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Cackovic, J.; Gutierrez-Luke, S.; Call, G.B.; Juba, A.; O’Brien, S.; Jun, C.H.; Buhlman, L.M. Vulnerable Parkin Loss-of-Function Drosophila Dopaminergic Neurons Have Advanced Mitochondrial Aging, Mitochondrial Network Loss and Transiently Reduced Autophagosome Recruitment. Front. Cell. Neurosci. 2018, 12, 39. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef]
- Delaidelli, A.; Richner, M.; Jiang, L.; van der Laan, A.; Bergholdt Jul Christiansen, I.; Ferreira, N.; Nyengaard, J.R.; Vægter, C.B.; Jensen, P.H.; Mackenzie, I.R.; et al. α-Synuclein pathology in Parkinson disease activates homeostatic NRF2 anti-oxidant response. Acta Neuropathol. Commun. 2021, 9, 105. [Google Scholar] [CrossRef]
- Todorovic, M.; Wood, S.A.; Mellick, G.D. Nrf2: A modulator of Parkinson’s disease? J. Neural. Transm. 2016, 123, 611–619. [Google Scholar] [CrossRef]
- Jenner, P.; Schapira, A.; Marsden, C. New insights into the cause of Parkinson’s disease. Neurology 1992, 42, 2241. [Google Scholar] [CrossRef]
- Müftüoglu, M.; Elibol, B.; Dalmızrak, Ö.; Ercan, A.; Kulaksız, G.; Ögüs, H.; Dalkara, T.; Özer, N. Mitochondrial complex I and IV activities in leukocytes from patients with parkin mutations. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, 544–548. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, H.Y.; Li, B.; Niu, G.Z.; Chen, S.D. Downregulation of parkin damages antioxidant defenses and enhances proteasome inhibition-induced toxicity in PC12 cells. J. Neuroimmune Pharmacol. 2007, 2, 276–283. [Google Scholar] [CrossRef]
- Mortiboys, H.; Thomas, K.; Koopman, W.; Klaffke, S.; Abou-Sleiman, P.; Olpin, S.; Wood, N.; Willems, P.; Smeitink, J.; Cookson, M. Mitochondrial function and morphology are impaired in parkin mutant fibroblasts. Ann. Neurol. 2008, 64, 555. [Google Scholar] [CrossRef]
- Srivastav, S.; Singh, S.K.; Yadav, A.K.; Srikrishna, S. Folic acid supplementation rescues anomalies associated with knockdown of parkin in dopaminergic and serotonergic neurons in Drosophila model of Parkinson’s disease. Biochem. Biophys. Res. Commun. 2015, 460, 780–785. [Google Scholar] [CrossRef]
- Gutscher, M.; Sobotta, M.C.; Wabnitz, G.H.; Ballikaya, S.; Meyer, A.J.; Samstag, Y.; Dick, T.P. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J. Biol. Chem. 2009, 284, 31532–31540. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, S.C.; Barata, A.G.; Grosshans, J.; Teleman, A.A.; Dick, T.P. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 2011, 14, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Gutscher, M.; Pauleau, A.-L.; Marty, L.; Brach, T.; Wabnitz, G.H.; Samstag, Y.; Meyer, A.J.; Dick, T.P. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 2008, 5, 553. [Google Scholar] [CrossRef]
- Child, D.F.; Hudson, P.R.; Jones, H.; Davies, G.K.; De, P.; Mukherjee, S.; Brain, A.M.; Williams, C.P.; Harvey, J.N. The effect of oral folic acid on glutathione, glycaemia and lipids in Type 2 diabetes. Diabetes Nutr. Metab. 2004, 17, 95–102. [Google Scholar]
- Ho, P.I.; Ashline, D.; Dhitavat, S.; Ortiz, D.; Collins, S.C.; Shea, T.B.; Rogers, E. Folate deprivation induces neurodegeneration: Roles of oxidative stress and increased homocysteine. Neurobiol. Dis. 2003, 14, 32–42. [Google Scholar] [CrossRef]
- Jadavji, N.M.; Emmerson, J.T.; Shanmugalingam, U.; MacFarlane, A.J.; Willmore, W.G.; Smith, P.D. A genetic deficiency in folic acid metabolism impairs recovery after ischemic stroke. Exp. Neurol. 2018, 309, 14–22. [Google Scholar] [CrossRef]
- Singh, R.; Kanwar, S.S.; Sood, P.K.; Nehru, B. Beneficial effects of folic acid on enhancement of memory and antioxidant status in aged rat brain. Cell Mol. Neurobiol. 2011, 31, 83–91. [Google Scholar] [CrossRef]
- Ojeda, M.L.; Rua, R.M.; Nogales, F.; Díaz-Castro, J.; Murillo, M.L.; Carreras, O. The Benefits of Administering Folic Acid in Order to Combat the Oxidative Damage Caused by Binge Drinking in Adolescent Rats. Alcohol Alcohol. 2016, 51, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Alcalay, R.N.; Caccappolo, E.; Mejia-Santana, H.; Tang, M.X.; Rosado, L.; Orbe Reilly, M.; Ruiz, D.; Louis, E.D.; Comella, C.L.; Nance, M.A.; et al. Cognitive and motor function in long-duration PARKIN-associated Parkinson disease. JAMA Neurol. 2014, 71, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Greene, J.C.; Whitworth, A.J.; Kuo, I.; Andrews, L.A.; Feany, M.B.; Pallanck, L.J. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. USA 2003, 100, 4078. [Google Scholar] [CrossRef] [Green Version]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef]
- Buhlman, L.M.; Keoseyan, P.P.; Houlihan, K.; Juba, A.N. Measuring Mitochondrial Hydrogen Peroxide Levels and Glutathione Redox Equilibrium in Drosophila Neuron Subtypes Using Redox-Sensitive Fluorophores and 3D Imaging. In Mitochondrial Medicine; Springer: New York, NY, USA, 2021; pp. 113–127. [Google Scholar]
- ThermoFisher. Coomassie Plus (Bradford) Assay Kit INSTRUCTIONS. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2FMAN0011203_CoomassiePlus_Bradford_Asy_UG.pdf (accessed on 1 September 2022).
- Kim, Y.Y.; Um, J.H.; Yoon, J.H.; Kim, H.; Lee, D.Y.; Lee, Y.J.; Jee, H.J.; Kim, Y.M.; Jang, J.S.; Jang, Y.G. Assessment of mitophagy in mt-Keima Drosophila revealed an essential role of the PINK1-Parkin pathway in mitophagy induction in vivo. FASEB J. 2019, 33, 9742–9751. [Google Scholar] [CrossRef] [Green Version]
- Vincent, A.; Briggs, L.; Chatwin, G.F.J.; Emery, E.; Tomlins, R.; Oswald, M.; Middleton, C.A.; Evans, G.J.O.; Sweeney, S.T.; Elliott, C.J.H. parkin-induced defects in neurophysiology and locomotion are generated by metabolic dysfunction and not oxidative stress. Hum. Mol. Genet. 2012, 21, 1760–1769. [Google Scholar] [CrossRef]
- Roy, S.; Sable, P.; Khaire, A.; Randhir, K.; Kale, A.; Joshi, S. Effect of maternal micronutrients (folic acid and vitamin B12) and omega 3 fatty acids on indices of brain oxidative stress in the offspring. Brain Dev. 2014, 36, 219–227. [Google Scholar] [CrossRef]
- BroadInstitute. GTExPortal. Available online: https://www.gtexportal.org/home/gene/PARK2 (accessed on 3 October 2022).
- Fu, Y.; Paxinos, G.; Watson, C.; Halliday, G.M. The substantia nigra and ventral tegmental dopaminergic neurons from development to degeneration. J. Chem. Neuroanat. 2016, 76, 98–107. [Google Scholar] [CrossRef]
- Brichta, L.; Greengard, P. Molecular determinants of selective dopaminergic vulnerability in Parkinson’s disease: An update. Front. Neuroanat. 2014, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Wu, T.; Zhao, J.; Song, A.; Liu, H.; Xu, W.; Huang, G. Folic acid supplementation improves cognitive function by reducing the levels of peripheral inflammatory cytokines in elderly Chinese subjects with MCI. Sci. Rep. 2016, 6, 37486. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.Y.; Chiu, S.W.; Hong, K.S.; Saver, J.L.; Wu, Y.L.; Lee, J.D.; Lee, M.; Ovbiagele, B. Folic Acid in Stroke Prevention in Countries without Mandatory Folic Acid Food Fortification: A Meta-Analysis of Randomized Controlled Trials. J. Stroke 2018, 20, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Sigma-Aldrich. SAFETY DATA SHEET—Folic Acid. Available online: https://www.sigmaaldrich.com/US/en/sds/sigma/f7876 (accessed on 1 September 2022).
- Harvard, T.C.S.o.P.H. Folate (Folic Acid)—Vitamin B9. Available online: https://www.hsph.harvard.edu/nutritionsource/folic-acid/ (accessed on 15 August 2022).
- Gumeni, S.; Papanagnou, E.D.; Manola, M.S.; Trougakos, I.P. Nrf2 activation induces mitophagy and reverses Parkin/Pink1 knock down-mediated neuronal and muscle degeneration phenotypes. Cell Death Dis. 2021, 12, 671. [Google Scholar] [CrossRef]
- Morgan, B.; Sobotta, M.C.; Dick, T.P. Measuring EGSH) and H2O2 with roGFP2-based redox probes. Free Radic. Biol. Med. 2011, 51, 1943–1951. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houlihan, K.L.; Keoseyan, P.P.; Juba, A.N.; Margaryan, T.; Voss, M.E.; Babaoghli, A.M.; Norris, J.M.; Adrian, G.J.; Tovmasyan, A.; Buhlman, L.M. Folic Acid Improves Parkin-Null Drosophila Phenotypes and Transiently Reduces Vulnerable Dopaminergic Neuron Mitochondrial Hydrogen Peroxide Levels and Glutathione Redox Equilibrium. Antioxidants 2022, 11, 2068. https://doi.org/10.3390/antiox11102068
Houlihan KL, Keoseyan PP, Juba AN, Margaryan T, Voss ME, Babaoghli AM, Norris JM, Adrian GJ, Tovmasyan A, Buhlman LM. Folic Acid Improves Parkin-Null Drosophila Phenotypes and Transiently Reduces Vulnerable Dopaminergic Neuron Mitochondrial Hydrogen Peroxide Levels and Glutathione Redox Equilibrium. Antioxidants. 2022; 11(10):2068. https://doi.org/10.3390/antiox11102068
Chicago/Turabian StyleHoulihan, Katherine L., Petros P. Keoseyan, Amber N. Juba, Tigran Margaryan, Max E. Voss, Alexander M. Babaoghli, Justin M. Norris, Greg J. Adrian, Artak Tovmasyan, and Lori M. Buhlman. 2022. "Folic Acid Improves Parkin-Null Drosophila Phenotypes and Transiently Reduces Vulnerable Dopaminergic Neuron Mitochondrial Hydrogen Peroxide Levels and Glutathione Redox Equilibrium" Antioxidants 11, no. 10: 2068. https://doi.org/10.3390/antiox11102068