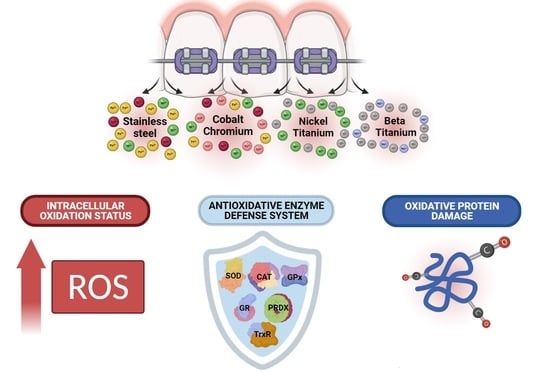
Causation of Oxidative Stress and Defense Response of a Yeast Cell Model after Treatment with Orthodontic Alloys Consisting of Metal Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Orthodontic Material Preparation
Metal Release and Alloy Constitution Analysis
2.2. Orthodontic Metal Ion Solutions and Yeast Metal Treatment
2.3. Preparation of Yeast Culture and Metal Treatment
2.4. Reactive Oxygen Species (ROS) Level Determination
2.5. Enzymatic Antioxidants Activity Determination
2.5.1. Cell Lysate Preparation
2.5.2. Superoxide Dismutase (Sod) Activity
2.5.3. Catalase (CAT) Activity
2.5.4. Glutathione Peroxidase (GPx) Activity
2.5.5. Glutathione Reductase (GR) Activity
2.5.6. Thioredoxin Reductase (TrxR) Activity
2.5.7. Peroxiredoxin (PRDX) Activity
2.5.8. In-Gel Enzyme Activity
2.6. Oxidative Protein Damages
2.7. Statistical Analysis
3. Results
3.1. Metal Ion Release
3.2. Intracellular Oxidation Level
3.3. Enzymatic Antioxidative Defense
3.4. Protein Oxidative Damage
4. Discussion
4.1. Topic: Metal Ion Release in a Simulated Environment
4.2. Topic: Metal Ion Exposure, Oxidative Stress, and the Enzymatic Antioxidant Defense System Induction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrett, R.D.; Bishara, S.E.; Quinn, J.K. Biodegradation of orthodontic appliances. Part I. Biodegradation of nickel and chromium in vitro. Am. J. Orthod. Dentofac. Orthop. 1993, 103, 8–14. [Google Scholar] [CrossRef]
- Moresca, R. Orthodontic treatment time: Can it be shortened? Dent. Press J. Orthod. 2018, 23, 90–105. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, A.J.; Fernández, E.; Vicente, A.; Calvo, J.L.; Ortiz, C. Metallic ions released from stainless steel, nickel-free, and titanium orthodontic alloys: Toxicity and DNA damage. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 115–122. [Google Scholar] [CrossRef]
- Keinan, D.; Mass, E.; Zilberman, U. Absorption of Nickel, Chromium, and Iron by the Root Surface of Primary Molars Covered with Stainless Steel Crowns. Int. J. Dent. 2010, 2010, 326124. [Google Scholar] [CrossRef] [Green Version]
- Maheshwari, M.; Verma, S.K.; Dhiman, S.K. Metal Hypersensitivity in Orthodontic Patient. J. Dent. Mater. Tech. 2015, 4, 111–115. [Google Scholar]
- Sifakakis, I.; Eliades, T. Adverse reactions to orthodontic materials. Aust. Dent. J. 2017, 62, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Kovač, V.; Poljšak, B.; Primožič, J.; Jamnik, P. Are Metal Ions That Make up Orthodontic Alloys Cytotoxic, and Do They Induce Oxidative Stress in a Yeast Cell Model? Int. J. Mol. Sci. 2020, 21, 7993. [Google Scholar] [CrossRef]
- Arregui, M.; Latour, F.; Gil, F.J.; Pérez, R.A.; Giner-Tarrida, L.; Delgado, L.M. Ion Release from Dental Implants, Prosthetic Abutments and Crowns under Physiological and Acidic Conditions. Coatings 2021, 11, 98. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- House, K.; Sernetz, F.; Dymock, D.; Sandy, J.R.; Ireland, A.J. Corrosion of orthodontic appliances—Should we care? Am. J. Orthod. Dentofac. Orthop. 2008, 133, 584–592. [Google Scholar] [CrossRef]
- Shi, X.; Dalal, N.S. Vanadate-Mediated Hydroxyl Radical Generation from Superoxide Radical in the Presence of NADH: Haber-Weiss vs Fenton Mechanism. Arch. Biochem. Biophys. 1993, 307, 336–341. [Google Scholar] [CrossRef]
- Desurmont, M. Carcinogenic effect of metals. Sem. Hop. 1983, 59, 2097–2099. [Google Scholar]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219. [Google Scholar]
- Park, H.S.; Kim, S.R.; Lee, Y.C. Impact of oxidative stress on lung diseases. Respirology 2009, 14, 27–38. [Google Scholar] [CrossRef]
- Prousek, J. Preparation and utilization of nano ZVI in Fenton-like reactions View project Fenton chemistry in biology and medicine*. Pure Appl. Chem 2007, 79, 2325–2338. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Vidossich, P.; Rovira, C. The reaction mechanisms of heme catalases: An atomistic view by ab initio molecular dynamics. Arch. Biochem. Biophys. 2012, 525, 121–130. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Hanna, P.M.; Mason, R.P. The Origin of the Hydroxyl Radical Oxygen in the Fenton Reaction. Free Radic. Biol. Med. 1997, 22, 885–888. [Google Scholar] [CrossRef]
- De Flora, S. Threshold mechanisms and site specificity in chromium(VI) carcinogenesis. Carcinogenesis 2000, 21, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Dalal, N.S.; Kasprzak, K.S. Generation of Free Radicals from Hydrogen Peroxide and Lipid Hydroperoxides in the Presence of Cr(III). Arch. Biochem. Biophys. 1993, 302, 294–299. [Google Scholar] [CrossRef]
- Leonard, S.; Gannett, P.M.; Rojanasakul, Y.; Schwegler-Berry, D.; Castranova, V.; Vallyathan, V.; Shi, X. Cobalt-mediated generation of reactive oxygen species and its possible mechanism. J. Inorg. Biochem. 1998, 70, 239–244. [Google Scholar] [CrossRef]
- Lu, H.; Shi, X.; Costa, M.; Huang, C. Carcinogenic effect of nickel compounds. Mol. Cell. Biochem. 2005, 279, 45–67. [Google Scholar] [CrossRef]
- Mendel, R.R.; Bittner, F. Cell biology of molybdenum. Biochim. Biophys. Acta—Mol. Cell Res. 2006, 1763, 621–635. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Liu, P.; Wang, L.; Luo, J.; Zhang, C.; Guo, X.; Hu, G.; Cao, H. Mitochondrial oxidative stress-induced hepatocyte apoptosis reflects increased molybdenum intake in caprine. Biol. Trace Elem. Res. 2016, 170, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Fagali, N.S.; Grillo, C.A.; Puntarulo, S.; Fernández, M.; De Mele, L. Biodegradation of metallic biomaterials: Its relation with the generation of reactive oxygen species Impact on rural population of agrochemicals used in transgenic crops in Argentina View project Eradication of burst release of copper ions from copper-bea. In Reactive Oxygen Species, Lipid Peroxidation and Protein Oxidation; Catalá, A., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2015; ISBN 9781633218864. [Google Scholar]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.; Zink, J.; Nel, A. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [Green Version]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Tóthová, L.; Celec, P. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Front. Physiol. 2017, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Tóthová, L.; Kamodyová, N.; Červenka, T.; Celec, P. Salivary markers of oxidative stress in oral diseases. Front. Cell. Infect. Microbiol. 2015, 5, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mesa-Herrera, F.; Quinto-Alemany, D.; Díaz, M. A Sensitive, Accurate, and Versatile Method for the Quantification of Superoxide Dismutase Activities in Biological Preparations. React. Oxyg. Species 2019, 7, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.D.; Levander, O.A. High-throughput 96-well microplate assays for determining specific activities of glutathione peroxidase and thioredoxin reductase. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2002; Volume 347, pp. 113–121. [Google Scholar]
- Glippa, O.; Engström-Öst, J.; Kanerva, M.; Rein, A.; Vuori, K. Oxidative stress and antioxidant defense responses in Acartia copepods in relation to environmental factors. PLoS ONE 2018, 13, e0195981. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.K.; Hadwan, M.H. Precise Spectrophotometric Method for measurement of Peroxiredoxin activity in Biological Samples. Res. J. Pharm. Technol. 2019, 12, 2254. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Keston, A.S.; Brandt, R. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal. Biochem. 1965, 11, 1–5. [Google Scholar] [CrossRef]
- Sousa, C.A.; Soares, H.M.V.M.; Soares, E.V. Nickel Oxide (NiO) Nanoparticles Induce Loss of Cell Viability in Yeast Mediated by Oxidative Stress. Chem. Res. Toxicol. 2018, 31, 658–665. [Google Scholar] [CrossRef]
- Breeuwer, P.; Drocourt, J.L.; Bunschoten, N.; Zwietering, M.H.; Rombouts, F.M.; Abee, T. Characterization of uptake and hydrolysis of fluorescein diacetate and carboxyfluorescein diacetate by intracellular esterases in Saccharomyces cerevisiae, which result in accumulation of fluorescent product. Appl. Environ. Microbiol. 1995, 61, 1614. [Google Scholar] [CrossRef] [Green Version]
- Valiakhmetov, A.Y.; Kuchin, A.V.; Suzina, N.E.; Zvonarev, A.N.; Shepelyakovskaya, A.O. Glucose causes primary necrosis in exponentially grown yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2019, 19, 19. [Google Scholar] [CrossRef]
- Tanaka, K.; Shimakawa, G.; Nakanishi, S. Time-of-day-dependent responses of cyanobacterial cellular viability against oxidative stress. Sci. Rep. 2020, 10, 20029. [Google Scholar] [CrossRef]
- Faccioni, F.; Franceschetti, P.; Cerpelloni, M.; Fracasso, M.E. In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 687–693. [Google Scholar] [CrossRef]
- Santos Genelhu, M.C.L.; Marigo, M.; Alves-Oliveira, L.F.; Cotta Malaquias, L.C.; Gomez, R.S. Characterization of nickel-induced allergic contact stomatitis associated with fixed orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2005, 128, 378–381. [Google Scholar] [CrossRef]
- Galeotti, A.; Uomo, R.; Spagnuolo, G.; Paduano, S.; Cimino, R.; Valletta, R.; D’Antò, V. Effect of pH on in vitro biocompatibility of orthodontic miniscrew implants. Prog. Orthod. 2013, 14, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials, 3rd ed.; Academic Press Inc.: Cambridge, MA, USA, 2013; pp. 1–1555. [Google Scholar]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.; Rodriguez, A.; Chang, D. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Urban, R.; Jacobs, J.; Gilbert, J.; Galante, J. Migration of corrosion products from modular hip prostheses. Particle microanalysis and histopathological findings. J. Bone Jt. Surg. Am. 1994, 76, 1345–1359. [Google Scholar] [CrossRef]
- Brantley, W.A.; Eliades, T. Orthodontic Materials: Scientific and Clinical Aspects; Thieme: Stuttgart, Germany, 2001; ISBN 9780865779297. [Google Scholar]
- Eliades, T. Orthodontic materials research and applications: Part 2. Current status and projected future developments in materials and biocompatibility. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 253–262. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Lobner, D. In vitro cytotoxicity of orthodontic archwires in cortical cell cultures. Eur. J. Orthod. 2004, 26, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Dentaurum Materials for Orthodontic Products. Available online: https://www.dentaurum.de/files/KFO-Werkstoffliste-20.pdf (accessed on 27 August 2020).
- Ramazanzadeh, B.A.; Ahrari, F.; Sabzevari, B.; Habibi, S. Original Articles Nickel Ion Release from Three Types of Nickel-titanium-based Orthodontic Archwires in the As-received State and After Oral Simulation. Dent. Clin. Dent. Prospect. J. Dent. Res. Dent. Clin. Dent. Prospect 2014, 8, 71–76. [Google Scholar] [CrossRef]
- Hunt, N.P.; Cunningham, S.J.; Golden, C.G.; Sheriff, M. An investigation into the effects of polishing on surface hardness and corrosion of orthodontic archwires. Angle Orthod. 1999, 69, 433–440. [Google Scholar] [CrossRef]
- Tsang, H. EU Harmonises Test Methods for Nickel Release under REACH. Available online: https://www.sgs.com/en/news/2016/01/safeguards-02216-eu-harmonises-test-methods-for-nickel-release-under-reach (accessed on 20 April 2020).
- Charles, A.; Gangurde, P.; Jacob, S.; Jatol-Tekade, S.; Senkutvan, R.; Vadgaonkar, V. Evaluation of nickel ion release from various orthodontic arch wires: An in vitro study. J. Int. Soc. Prev. Community Dent. 2014, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Hussain, H.D.; Ajith, S.D.; Goel, P. Nickel release from stainless steel and nickel titanium archwires-An in vitro study. J. Oral Biol. Craniofacial Res. 2016, 6, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez, C.; Vilar, T.; Sevilla, P.; Gil, J. In vitro corrosion behaviour of lingual orthodontic archwires. Int. J. Corros. 2011, 2011, 482485. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-H.; Wang, C.-C.; Chiu, S.-M.; Wang, J.-F.; Liaw, Y.-C.; Lee, T.-H.; Chen, F.-L. Corrosion Behavior of titanium-containing Orthodontic Archwires in Artificial Saliva: Effects of Fluoride Ions and Plasma Immersion Ion Implantation Treatment. J. Dent. Sci. 2005, 24, 134–140. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Hwang, C.J.; Shin, J.S.; Cha, J.Y. Metal release from simulated fixed orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2001, 120, 383–391. [Google Scholar] [CrossRef]
- Hussain, S.; Asshaari, A.; Osman, B.; AL-Bayaty, F. In Vitro-Evaluation of Biodegradation of Different Metallic Orthodontic Brackets. J. Int. Dent. Med. Res. 2017, 7, 76–83. [Google Scholar]
- Mikulewicz, M.; Chojnacka, K.; Woźniak, B.; Downarowicz, P. Release of metal ions from orthodontic appliances: An in vitro study. Biol. Trace Elem. Res. 2012, 146, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Kuhta, M.; Pavlin, D.; Slaj, M.M.; Varga, S.; Lapter-Varga, M.; Slaj, M.M. Type of archwire and level of acidity: Effects on the release of metal ions from orthodontic appliances. Angle Orthod. 2009, 79, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staffolani, N.; Damiani, F.; Lilli, C.; Guerra, M.; Staffolani, N.J.; Beicastro, S.; Locci, P. Ion release from orthodontic appliances. J. Dent. 1999, 27, 449–454. [Google Scholar] [CrossRef]
- Wendl, B.; Wiltsche, H.; Lankmayr, E.; Winsauer, H.; Walter, A.; Muchitsch, A.; Jakse, N.; Wendl, M.; Wendl, T. Metal release profiles of orthodontic bands, brackets, and wires: An in vitro study. J. Orofac. Orthop. Fortschr. Kieferorthopädie 2017, 78, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Eliades, T.; Pratsinis, H.; Kletsas, D.; Eliades, G.; Makou, M. Characterization and cytotoxicity of ions released from stainless steel and nickel-titanium orthodontic alloys. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Spalj, S.; Mlacovic Zrinski, M.; Tudor Spalj, V.; Ivankovic Buljan, Z. In-vitro assessment of oxidative stress generated by orthodontic archwires. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Rincic Mlinaric, M.; Durgo, K.; Katic, V.; Spalj, S. Cytotoxicity and oxidative stress induced by nickel and titanium ions from dental alloys on cells of gastrointestinal tract. Toxicol. Appl. Pharmacol. 2019, 383, 114784. [Google Scholar] [CrossRef]
- Pallero, M.A.; Roden, M.T.; Chen, Y.-F.; Anderson, P.G.; Lemons, J.; Brott, B.C.; Murphy-Ullrich, J.E. Stainless Steel Ions Stimulate Increased Thrombospondin-1-Dependent TGF-Beta Activation by Vascular Smooth Muscle Cells: Implications for In-Stent Restenosis. J. Vasc. Res. 2010, 47, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Salloum, Z.; Lehoux, E.A.; Harper, M.E.; Catelas, I. Effects of cobalt and chromium ions on oxidative stress and energy metabolism in macrophages in vitro. J. Orthop. Res. 2018, 36, 3178–3187. [Google Scholar] [CrossRef] [Green Version]
- Scharf, B.; Clement, C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.; Purdue, E.; Goldring, S.; Macaluso, F.; Cobelli, N.; et al. Molecular analysis of chromium and cobalt-related toxicity. Sci. Rep. 2014, 4, 5729. [Google Scholar] [CrossRef] [Green Version]
- Petit, A.; Mwale, F.; Tkaczyk, C.; Antoniou, J.; Zukor, D.; Huk, O. Induction of protein oxidation by cobalt and chromium ions in human U937 macrophages. Biomaterials 2005, 26, 4416–4422. [Google Scholar] [CrossRef] [PubMed]
- Terpilowska, S.; Siwicki, A.K. Pro- and antioxidant activity of chromium(III), iron(III), molybdenum(III) or nickel(II) and their mixtures. Chem. Biol. Interact. 2019, 298, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Sevcikova, M.; Modra, H.; Slaninova, A.; Svobodova, Z. Metals as a cause of oxidative stress in fish: A review. Vet. Med. 2011, 56, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Arthur, J. The glutathione peroxidases. Cell. Mol. Life Sci. 2000, 57, 1825–1835. [Google Scholar] [CrossRef]
- Nenkova, G.; Petrov, L.; Alexandrova, A. Role of Trace Elements for Oxidative Status and Quality of Human Sperm. Balk. Med. J. 2017, 34, 343. [Google Scholar] [CrossRef]
- Gromer, S.; Urig, S.; Becker, K. The thioredoxin system—From science to clinic. Med. Res. Rev. 2004, 24, 40–89. [Google Scholar] [CrossRef]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Franchi, N.; Bakiu, R.; Ballarin, L.; Santovito, G. Molecular characterization and metal induced gene expression of the novel glutathione peroxidase 7 from the chordate invertebrate Ciona robusta. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 205, 1–7. [Google Scholar] [CrossRef]
- Longo, V.D.; Gralla, E.B.; Valentine, J.S. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J. Biol. Chem. 1996, 271, 12275–12280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez, J.; González, B.; Sempere, V.; Mas, A.; Torija, M.J.; Beltran, G. Melatonin reduces oxidative stress damage induced by hydrogen peroxide in Saccharomyces cerevisiae. Front. Microbiol. 2017, 8, 1066. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Fabrizio, P. Chronological aging in Saccharomyces cerevisiae. Subcell. Biochem. 2012, 57, 101–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigler, K.; Chaloupka, J.; Brozmanová, J.; Stadler, N.; Höfer, M. Oxidative stress in microorganisms—I. Microbial vs. higher cells—damage and defenses in relation to cell aging and death. Folia Microbiol. 1999, 44, 587–624. [Google Scholar] [CrossRef] [PubMed]
- Lopert, P.; Day, B.J.; Patel, M. Thioredoxin Reductase Deficiency Potentiates Oxidative Stress, Mitochondrial Dysfunction and Cell Death in Dopaminergic Cells. PLoS ONE 2012, 7, e50683. [Google Scholar] [CrossRef] [Green Version]
- Agledal, L.; Niere, M.; Ziegler, M. The phosphate makes a difference: Cellular functions of NADP. Redox Rep. 2010, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atli, G.; Canli, M. Response of antioxidant system of freshwater fish Oreochromis niloticus to acute and chronic metal (Cd, Cu, Cr, Zn, Fe) exposures. Ecotoxicol. Environ. Saf. 2010, 73, 1884–1889. [Google Scholar] [CrossRef]
- Bayliak, M.; Semchyshyn, H.; Lushchak, V. Effect of hydrogen peroxide on antioxidant enzyme activities in Saccharomyces cerevisiae is strain-specific. Biochem. 2006, 71, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ruiz, M.; González-Gordo, S.; Cañas, A.; Campos, M.J.; Paradela, A.; Corpas, F.J.; Palma, J.M. Sweet Pepper (Capsicum annuum L.) Fruits Contain an Atypical Peroxisomal Catalase That Is Modulated by Reactive Oxygen and Nitrogen Species. Antioxidants 2019, 8, 374. [Google Scholar] [CrossRef] [Green Version]
- Kubrak, O.I.; Lushchak, O.V.; Lushchak, J.V.; Torous, I.M.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Chromium effects on free radical processes in goldfish tissues: Comparison of Cr(III) and Cr(VI) exposures on oxidative stress markers, glutathione status and antioxidant enzymes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 152, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Tandoğan, B.; Ulusu, N.N. The inhibition kinetics of yeast glutathione reductase by some metal ions. J. Enzym. Inhib. Med. Chem. 2008, 22, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Khalifaa, H.H.; Hadwana, M.H. Simple Method for the Assessment of Peroxiredoxin Activity in Biological Sample. Chem. Data Collect. 2020, 27, 100376. [Google Scholar] [CrossRef]
- Mitozo, P.A.; De Souza, L.F.; Loch-Neckel, G.; Flesch, S.; Maris, A.F.; Figueiredo, C.P.; Dos Santos, A.R.S.; Farina, M.; Dafre, A.L. A study of the relative importance of the peroxiredoxin-, catalase-, and glutathione-dependent systems in neural peroxide metabolism. Free Radic. Biol. Med. 2011, 51, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarova, N.; Krumova, E.; Stefanova, T.; Georgieva, N.; Angelova, M. The oxidative stress response of the filamentous yeast Trichosporon cutaneum R57 to copper, cadmium and chromium exposure. Biotechnol. Biotechnol. Equip. 2014, 28, 855–862. [Google Scholar] [CrossRef]
- Feng, M.; Yin, H.; Peng, H.; Liu, Z.; Lu, G.; Dang, Z. Hexavalent chromium induced oxidative stress and apoptosis in Pycnoporus sanguineus. Environ. Pollut. 2017, 228, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, P.; Saranya, S.; Poornima, P.; Prabhakaran, R.; Dallemer, F.; Vijaya Padma, V.; Natarajan, K. Biological evaluation of new nickel(II) metallates: Synthesis, DNA/protein binding and mitochondrial mediated apoptosis in human lung cancer cells (A549) via ROS hypergeneration and depletion of cellular antioxidant pool. Eur. J. Med. Chem. 2014, 82, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Saquib, Q.; Ahamed, M.; Farshori, N.N.; Ahmad, J.; Wahab, R.; Khan, S.T.; Alhadlaq, H.A.; Musarrat, J.; Al-Khedhairy, A.A.; et al. Molybdenum nanoparticles-induced cytotoxicity, oxidative stress, G2/M arrest, and DNA damage in mouse skin fibroblast cells (L929). Colloids Surfaces B Biointerfaces 2015, 125, 73–81. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Zhu, Y.; Zhang, Y. Interaction of chromium(III) or chromium(VI) with catalase and its effect on the structure and function of catalase: An in vitro study. Food Chem. 2018, 244, 378–385. [Google Scholar] [CrossRef]
- Back, D.; Young, D.; Shimmin, A. How do serum cobalt and chromium levels change after metal-on-metal hip resurfacing? Clin. Orthop. Relat. Res. 2005, 438, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Allan, D.G.; Parsley, B.; Dyrstad, B.; Trammell, R.; Milbrandt, J.C. Elevation of Serum Cobalt and Chromium Levels in Patients With Metal-On-Metal Resurfacing Hip Prostheses: A 3-Year Follow-up. J. Arthroplasty 2007, 22, 311. [Google Scholar] [CrossRef]
- Antoniou, J.; Zukor, D.; Mwale, F.; Minarik, W.; Petit, A.; Huk, O. Metal ion levels in the blood of patients after hip resurfacing: A comparison between twenty-eight and thirty-six-millimeter-head metal-on-metal prostheses. J. Bone Jt. Surg. Am. 2008, 90 (Suppl. 3), 142–148. [Google Scholar] [CrossRef]
- Fleury, C.; Petit, A.; Mwale, F.; Antoniou, J.; Zukor, D.J.; Tabrizian, M.; Huk, O.L. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: Morphology, cytotoxicity, and oxidative stress. Biomaterials 2006, 27, 3351–3360. [Google Scholar] [CrossRef]
- Wang, Y.; Su, H.; Gu, Y.; Song, X.; Zhao, J. Carcinogenicity of chromium and chemoprevention: A brief update. Onco Targets Ther. 2017, 10, 4065–4079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magone, K.; Luckenbill, D.; Goswami, T. Metal ions as inflammatory initiators of osteolysis. Arch. Orthop. Trauma Surg. 2015, 135, 683–695. [Google Scholar] [CrossRef]
- Baskey, S.; Lehoux, E.; Catelas, I. Effects of cobalt and chromium ions on lymphocyte migration. J. Orthop. Res. 2017, 35, 916–924. [Google Scholar] [CrossRef] [Green Version]
- Buczko, P.; Knaś, M.; Grycz, M.; Szarmach, I.; Zalewska, A. Orthodontic treatment modifies the oxidant–antioxidant balance in saliva of clinically healthy subjects. Adv. Med. Sci. 2017, 62, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, A.M.; Martinez, E.F.; Demasi, A.P.D. Evaluation of toxicity and response to oxidative stress generated by orthodontic bands in human gingival fibroblasts. Angle Orthod. 2020, 90, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Scarcello, E.; Herpain, A.; Tomatis, M.; Turci, F.; Jacques, P.J.; Lison, D. Hydroxyl radicals and oxidative stress: The dark side of Fe corrosion. Colloids Surfaces B Biointerfaces 2020, 185, 110542. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Fagali, N.S.; Grillo, C.A.; Puntarulo, S.; Fernández Lorenzo de Mele, M.A. Is there any difference in the biological impact of soluble and insoluble degradation products of iron-containing biomaterials? Colloids Surfaces B Biointerfaces 2017, 160, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Kim, M.K.; Leesungbok, R.; Lee, S.W.; Ahn, S.J. Co–Cr dental alloys induces cytotoxicity and inflammatory responses via activation of Nrf2/antioxidant signaling pathways in human gingival fibroblasts and osteoblasts. Dent. Mater. 2016, 32, 1394–1405. [Google Scholar] [CrossRef]
- Marti, A. Cobalt-base alloys used in bone surgery. Injury 2000, 31, D18–D21. [Google Scholar] [CrossRef]
- Bazaka, O.; Bazaka, K.; Kingshott, P.; Crawford, R.J.; Ivanova, E.P. Chapter 1 Metallic Implants for Biomedical Applications. Chem. Inorg. Biomater. 2021, 8, 1–98. [Google Scholar] [CrossRef]
- Pohler, O.E.M. Unalloyed titanium for implants in bone surgery. Injury 2000, 31, D7–D13. [Google Scholar] [CrossRef]






| Component | Type | Specification | Parts in the Sample | Combined Surface (cm2) | Fe (%) | Ni (%) | Cr (%) | Co (%) | Mo (%) | Ti (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Archwire | Stainless steel | Damon 0.016 × 0.25 Ormco, Brea, CA, USA | 2 | 5.104 | 58.3 | 14.6 | 27.1 | <0.1 | <0.1 | <0.1 |
| Ni-Ti | Biostarter 0.016” Forestadent, Pforzheim, Germany | 2 | 4.172 | <0.1 | 73.8 | <0.1 | <0.1 | <0.1 | 26.2 | |
| Ni-Ti | rematitan super elastic 0.016” Dentaurum, Ispringen, Germany | 2 | 4.149 | <0.1 | 73.2 | <0.1 | <0.1 | <0.1 | 26.8 | |
| Ti-Mo | rematitan SPECIAL 0.032” Dentaurum, Ispringen, Germany | 2 | 6.846 | <0.1 | <0.1 | <0.1 | <0.1 | 8.18 | 91.8 | |
| Co-Cr-Ni | Elgiloy 0.036” Rocky Mountain Orthodontics, Denver, CO, USA | 2 | 9.476 | 6.12 | 19.9 | 21.6 | 49.7 | 2.65 | <0.1 | |
| Co-Cr-Ni | remaloy.036” Dentaurum, Ispringen, Germany | 2 | 9.217 | 1.42 | 26.1 | 19.4 | 53.2 | <0.1 | <0.1 | |
| Brackets | Stainless steel | Discovery Dentaurum, Ispringen, Germany | 24 | 14.478 | 55.4 | 17.8 | 24.9 | <0.1 | 1.95 | <0.1 |
| Molar bands | Stainless steel | W-Fit Form Forestadent, Pforzheim, Germany | 4 | 9.881 | 57 | 18.2 | 24.9 | <0.1 | <0.1 | <0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovač, V.; Bergant, M.; Ščančar, J.; Primožič, J.; Jamnik, P.; Poljšak, B. Causation of Oxidative Stress and Defense Response of a Yeast Cell Model after Treatment with Orthodontic Alloys Consisting of Metal Ions. Antioxidants 2022, 11, 63. https://doi.org/10.3390/antiox11010063
Kovač V, Bergant M, Ščančar J, Primožič J, Jamnik P, Poljšak B. Causation of Oxidative Stress and Defense Response of a Yeast Cell Model after Treatment with Orthodontic Alloys Consisting of Metal Ions. Antioxidants. 2022; 11(1):63. https://doi.org/10.3390/antiox11010063
Chicago/Turabian StyleKovač, Vito, Matic Bergant, Janez Ščančar, Jasmina Primožič, Polona Jamnik, and Borut Poljšak. 2022. "Causation of Oxidative Stress and Defense Response of a Yeast Cell Model after Treatment with Orthodontic Alloys Consisting of Metal Ions" Antioxidants 11, no. 1: 63. https://doi.org/10.3390/antiox11010063