Comparison of Antioxidant Properties of a Conjugate of Taxifolin with Glyoxylic Acid and Selected Flavonoids
Abstract
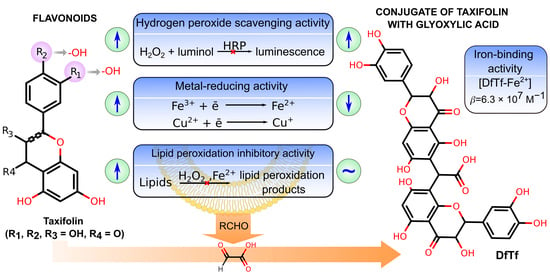
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Hydrogen Peroxide Scavenging Activity
2.3. Metal-Reducing Activity
2.3.1. Copper-Reducing Activity
2.3.2. Iron-Reducing Activity
2.4. Iron-Binding Properties of DfTf
2.5. Lipid Peroxidation Inhibitory Activity
2.6. Statistical Analysis
3. Results
3.1. Hydrogen Peroxide Scavenging Activity
3.2. Metal-Reducing Activity
3.3. Iron-Binding Properties of DfTf
3.4. Lipid Peroxidation Inhibitory Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Gould, K.S.; Lister, C. Flavonoid functions in plants. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, O.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; p. 1237. ISBN 978-0-8493-2021-7. [Google Scholar]
- Mathesius, U. Flavonoid functions in plants and their interactions with other organisms. Plants 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 419–441. ISBN 978-0-12-822919-4. [Google Scholar]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse Association between habitual polyphenol intake and incidence of cardiovascular events in the predimed study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Johanna Rienks; Janett Barbaresko; Ute nöthlings association of polyphenol biomarkers with cardiovascular disease and mortality risk: A systematic review and meta-analysis of observational studies. Nutrients 2017, 9, 415. [CrossRef] [Green Version]
- Lindsay, J. Risk factors for alzheimer’s disease: A prospective analysis from the Canadian study of health and aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.B.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef]
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.; Barberger-Gateau, P. Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar] [CrossRef]
- Commenges, D.; Scotet, V.; Renaud, S.; Jacqmin-Gadda, H.; Barberger-Gateau, P.; Dartigues, J.-F. Intake of flavonoids and risk of dementia. Eur. J. Epidemiol. 2000, 16, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetz, M.E.; Judd, S.E.; Hartman, T.J.; McClellan, W.; Anderson, A.; Vaccarino, V. Flavanone intake is inversely associated with risk of incident ischemic stroke in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. J. Nutr. 2016, 146, 2233–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Es-Safi, N.-E.; Cheynier, V.; Moutounet, M. Study of the reactions between (+)-catechin and furfural derivatives in the presence or absence of anthocyanins and their implication in food color change. J. Agric. Food Chem. 2000, 48, 5946–5954. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Cheynier, V.; Moutounet, M. Role of aldehydic derivatives in the condensation of phenolic compounds with emphasis on the sensorial properties of fruit-derived foods. J. Agric. Food Chem. 2002, 50, 5571–5585. [Google Scholar] [CrossRef]
- Lo, C.-Y.; Li, S.; Tan, D.; Pan, M.-H.; Sang, S.; Ho, C.-T. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol. Nutr. Food Res. 2006, 50, 1118–1128. [Google Scholar] [CrossRef]
- Totlani, V.M.; Peterson, D.G. Epicatechin carbonyl-trapping reactions in aqueous maillard systems: Identification and structural elucidation. J. Agric. Food Chem. 2006, 54, 7311–7318. [Google Scholar] [CrossRef]
- Liu, G.; Xia, Q.; Lu, Y.; Zheng, T.; Sang, S.; Lv, L. Influence of quercetin and its methylglyoxal adducts on the formation of α-dicarbonyl compounds in a lysine/glucose model system. J. Agric. Food Chem. 2017, 65, 2233–2239. [Google Scholar] [CrossRef]
- Delgado, R.M.; Hidalgo, F.J.; Zamora, R. Antagonism between lipid-derived reactive carbonyls and phenolic compounds in the strecker degradation of amino acids. Food Chem. 2016, 194, 1143–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo, F.J.; Delgado, R.M.; Zamora, R. Protective effect of phenolic compounds on carbonyl-amine reactions produced by lipid-derived reactive carbonyls. Food Chem. 2017, 229, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Zamora, R.; Aguilar, I.; Hidalgo, F.J. Epoxyalkenal-trapping ability of phenolic compounds. Food Chem. 2017, 237, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. The stomach as a “bioreactor”: When red meat meets red wine. J. Agric. Food Chem. 2008, 56, 5002–5007. [Google Scholar] [CrossRef]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. A Novel function of red wine polyphenols in humans: Prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2008, 22, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by polyphenols of postprandial human plasma and low-density lipoprotein modification: The stomach as a bioreactor. J. Agric. Food Chem. 2012, 60, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Sirota, R.; Gorelik, S.; Harris, R.; Kohen, R.; Kanner, J. Coffee polyphenols protect human plasma from postprandial carbonyl modifications. Mol. Nutr. Food Res. 2013, 57, 916–919. [Google Scholar] [CrossRef]
- Kanner, J.; Selhub, J.; Shpaizer, A.; Rabkin, B.; Shacham, I.; Tirosh, O. Redox homeostasis in stomach medium by foods: The postprandial oxidative stress index (posi) for balancing nutrition and human health. Redox Biol. 2017, 12, 929–936. [Google Scholar] [CrossRef]
- Shubina, V.S.; Shatalin, Y.V. Antioxidant and iron-chelating properties of taxifolin and its condensation product with glyoxylic acid. J. Food Sci. Technol. 2017, 54, 1467–1475. [Google Scholar] [CrossRef] [Green Version]
- Mira, L.; Tereza Fernandez, M.; Santos, M.; Rocha, R.; Helena Florêncio, M.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Shatalin, I.V.; Shmarev, A.N. Peroxidation of lecithin in the presence of dihydroquercetin and its complex with ferrous iron ions. Biofizika 2010, 55, 75–82. [Google Scholar]
- Cai, Y.-Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Structure-activity relationships analysis of monomeric and polymeric polyphenols (quercetin, rutin and catechin) obtained by various polymerization methods. Chem. Biodivers. 2019, 16. [Google Scholar] [CrossRef]
- Hashemi Gahruie, H.; Niakousari, M. Antioxidant, antimicrobial, cell viability and enzymatic inhibitory of antioxidant polymers as biological macromolecules. Int. J. Biol. Macromol. 2017, 104, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.E.; Kurisawa, M.; Kim, Y.-J.; Uyama, H.; Kobayashi, S. Amplification of antioxidant activity of catechin by polycondensation with acetaldehyde. Biomacromolecules 2004, 5, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Chung, J.E.; Kurisawa, M.; Uyama, H.; Kobayashi, S. Superoxide anion scavenging and xanthine oxidase inhibition of (+)-catechin-aldehyde polycondensates. amplification of the antioxidant property of (+)-catechin by polycondensation with aldehydes. Biomacromolecules 2004, 5, 547–552. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Uyama, H.; Kobayashi, S. Inhibition effects of (+)-catechin–aldehyde polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261. [Google Scholar] [CrossRef]
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S. Enzymatic synthesis and antioxidant properties of poly(rutin). Biomacromolecules 2003, 4, 1394–1399. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A.; Piotrowska, M. Thermally stable and antimicrobial active poly(catechin) obtained by reaction with a cross-linking agent. Biomolecules 2020, 11, 50. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A.; Piotrowska, M. Novel polymeric biomaterial based on naringenin. Materials 2021, 14, 2142. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Boulaaba, M.; Guyot, S.; Abdelly, C.; Magné, C. Phenolic nature, occurrence and polymerization degree as marker of environmental adaptation in the edible halophyte mesembryanthemum edule. S. Afr. J. Bot. 2012, 79, 117–124. [Google Scholar] [CrossRef]
- Jerez, M.; Touriño, S.; Sineiro, J.; Torres, J.L.; Núñez, M.J. Procyanidins from pine bark: Relationships between structure, composition and antiradical activity. Food Chem. 2007, 104, 518–527. [Google Scholar] [CrossRef]
- Spranger, I.; Sun, B.; Mateus, A.M.; de Freitas, V.; Ricardo-da-Silva, J.M. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008, 108, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.-C.; Tam, N.F.; Lin, Y.-M.; Ding, Z.-H.; Chai, W.-M.; Wei, S.-D. Relationships between degree of polymerization and antioxidant activities: A study on proanthocyanidins from the leaves of a medicinal mangrove plant ceriops tagal. PLoS ONE 2014, 9, e107606. [Google Scholar] [CrossRef]
- Jomová, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A switch between antioxidant and prooxidant properties of the phenolic compounds myricetin, morin, 3′,4′-dihydroxyflavone, taxifolin and 4-hydroxy-coumarin in the presence of copper(ii) ions: A spectroscopic, absorption titration and dna damage study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. prooxidant properties of the flavonoid, kaempferol, in the presence of cu(ii) ions: A ros-scavenging activity, fenton reaction and dna damage study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef] [PubMed]
- Shubina, V.S.; Shatalina, Y.V. Absorption spectroscopy study of acid-base and metal-binding properties of flavanones. J. Appl. Spectrosc. 2013, 80, 761–766. [Google Scholar] [CrossRef]
- Shubina, V.S.; Kozina, V.I.; Shatalin, Y.V. Effect of complex formation by taxifolin and naringenin with cu(i) ions on the distribution of the components of complexes in the octanol–water system. Russ. J. Bioorganic Chem. 2017, 43, 463–470. [Google Scholar] [CrossRef]
- Mladěnka, P.; Macáková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef]
- Fernandez, M.T.; Mira, M.L.; Florêncio, M.H.; Jennings, K.R. Iron and copper chelation by flavonoids: An electrospray mass spectrometry study. J. Inorg. Biochem. 2002, 92, 105–111. [Google Scholar] [CrossRef]
- Guo, M.; Perez, C.; Wei, Y.; Rapoza, E.; Su, G.; Bou-Abdallah, F.; Chasteen, N.D. Iron-binding properties of plant phenolics and cranberry’s bio-effects. Dalton Trans. 2007, 4951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, C.R. Characterization and application of ferrozine iron reagent as a ferrous iron indicator. Anal. Chem. 1976, 48, 1197–1201. [Google Scholar] [CrossRef]
- Xiao, Z.; Brose, J.; Schimo, S.; Ackland, S.M.; La Fontaine, S.; Wedd, A.G. Unification of the copper(i) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins. J. Biol. Chem. 2011, 286, 11047–11055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias, J.; Pazos, M.; Lois, S.; Medina, I. Contribution of galloylation and polymerization to the antioxidant activity of polyphenols in fish lipid systems. J. Agric. Food Chem. 2010, 58, 7423–7431. [Google Scholar] [CrossRef] [Green Version]
- Perez, C.A.; Wei, Y.; Guo, M. Iron-binding and anti-fenton properties of baicalein and baicalin. J. Inorg. Biochem. 2009, 103, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Catapano, M.; Tvrdý, V.; Karlíčková, J.; Migkos, T.; Valentová, K.; Křen, V.; Mladěnka, P. The stoichiometry of isoquercitrin complex with iron or copper is highly dependent on experimental conditions. Nutrients 2017, 9, 1193. [Google Scholar] [CrossRef] [Green Version]
- Lomozová, Z.; Catapano, M.C.; Hrubša, M.; Karlíčková, J.; Macáková, K.; Kučera, R.; Mladěnka, P. Chelation of iron and copper by quercetin b-ring methyl metabolites, isorhamnetin and tamarixetin, and their effect on metal-based fenton chemistry. J. Agric. Food Chem. 2021, 69, 5926–5937. [Google Scholar] [CrossRef]
- Lopes, G.K.B.; Schulman, H.M.; Hermes-Lima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from fenton reaction by complexing ferrous ions1this study is dedicated to the memory of botany professor Luiz, F.G. Labouriau (1921–1996).1. Biochim. Biophys. Acta BBA Gen. Subj. 1999, 1472, 142–152. [Google Scholar] [CrossRef]
- Macáková, K.; Mladěnka, P.; Filipský, T.; Říha, M.; Jahodář, L.; Trejtnar, F.; Bovicelli, P.; Proietti Silvestri, I.; Hrdina, R.; Saso, L. Iron reduction potentiates hydroxyl radical formation only in flavonols. Food Chem. 2012, 135, 2584–2592. [Google Scholar] [CrossRef]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta BBA Gen. Subj. 2005, 1721, 174–184. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Říha, M.; Karlíčková, J.; Filipský, T.; Macáková, K.; Rocha, L.; Bovicelli, P.; Silvestri, I.P.; Saso, L.; Jahodář, L.; Hrdina, R.; et al. In vitro evaluation of copper-chelating properties of flavonoids. RSC Adv. 2014, 4, 32628–32638. [Google Scholar] [CrossRef] [Green Version]
- Saija, A.; Scalese, M.; Lanza, M.; Marzullo, D.; Bonina, F.; Castelli, F. Flavonoids as antioxidant agents: Importance of their interaction with biomembranes. Free Radic. Biol. Med. 1995, 19, 481–486. [Google Scholar] [CrossRef]
- Chen, X.; Ahn, D.U. Antioxidant activities of six natural phenolics against lipid oxidation induced by Fe2+ or ultraviolet light. J. Am. Oil Chem. Soc. 1998, 75, 1717. [Google Scholar] [CrossRef]
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.-S.; Lee, I.-K.; Kim, J.-P.; Chung, S.-H.; Shim, G.-S.; Yoo, I.-D. Lipid peroxidation inhibitory activity of some constituents isolated from the stem bark ofeucalyptus globulus. Arch. Pharm. Res. 2000, 23, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; Kelly, M.F. Inhibition of lipid peroxidation by anthocyanins, anthocyanidins and their phenolic degradation products. Eur. J. Lipid Sci. Technol. 2007, 109, 66–71. [Google Scholar] [CrossRef]
- Foti, M.; Piattelli, M.; Baratta, M.T.; Ruberto, G. Flavonoids, coumarins, and cinnamic acids as antioxidants in a micellar system. structure−activity relationship †. J. Agric. Food Chem. 1996, 44, 497–501. [Google Scholar] [CrossRef]
- Zu, Y.; Wu, W.; Zhao, X.; Li, Y.; Zhong, C.; Zhang, Y. The high water solubility of inclusion complex of taxifolin-γ-cd prepared and characterized by the emulsion solvent evaporation and the freeze drying combination method. Int. J. Pharm. 2014, 477, 148–158. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. EFSA panel on dietetic products, nutrition and allergies (nda). scientific opinion on taxifolin-rich extract from Dahurian Larch (Larix Gmelinii). EFSA J. 2017, 15, e04682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R.A. Experimental determination of octanol−water partition coefficients of quercetin and related flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef] [PubMed]
- Günther, G.; Berríos, E.; Pizarro, N.; Valdés, K.; Montero, G.; Arriagada, F.; Morales, J. Flavonoids in Microheterogeneous Media, relationship between their relative location and their reactivity towards singlet oxygen. PLoS ONE 2015, 10, e0129749. [Google Scholar] [CrossRef] [Green Version]
- Cuevas-Valenzuela, J.; González-Rojas, Á.; Wisniak, J.; Apelblat, A.; Pérez-Correa, J.R. Solubility of (+)-catechin in water and water-ethanol mixtures within the temperature range 277.6–331.2k: Fundamental data to design polyphenol extraction processes. Fluid Phase Equilibria 2014, 382, 279–285. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J. Solubility of hesperetin in various solvents from (288.2 to 323.2) K. J. Chem. Eng. Data 2008, 53, 1649–1650. [Google Scholar] [CrossRef]
- Perrissoud, D.; Testa, B. Inhibiting or potentiating effects of flavonoids on carbon tetrachloride-induced toxicity in isolated rat hepatocytes. Arzneimittelforschung. 1986, 36, 1249–1253. [Google Scholar]
- Sangpheak, W.; Kicuntod, J.; Schuster, R.; Rungrotmongkol, T.; Wolschann, P.; Kungwan, N.; Viernstein, H.; Mueller, M.; Pongsawasdi, P. Physical properties and biological activities of hesperetin and naringenin in complex with methylated β-Cyclodextrin. Beilstein J. Org. Chem. 2015, 11, 2763–2773. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, S.; Nabekura, T.; Takahashi, T.; Nakamura, Y.; Sakamoto, H.; Tano, H.; Hirai, M.; Tsukahara, G. Structure-activity relationships of the inhibitory effects of flavonoids on p-glycoprotein-mediated transport in KB-C2 cells. Biol. Pharm. Bull. 2005, 28, 2274–2278. [Google Scholar] [CrossRef] [Green Version]
- Pazos, M.; Gallardo, J.M.; Torres, J.L.; Medina, I. Activity of grape polyphenols as inhibitors of the oxidation of fish lipids and frozen fish muscle. Food Chem. 2005, 92, 547–557. [Google Scholar] [CrossRef]






| Polyphenols | IC50, µM |
|---|---|
| DfTf | 0.48 ± 0.04 * |
| eriodictyol | 0.73 ± 0.05 |
| quercetin | 0.75 ± 0.05 |
| taxifolin | 0.76 ± 0.05 |
| catechin | 0.93 ± 0.09 |
| hesperetin | 2.17 ± 0.15 * |
| naringenin | 34.2 ± 4.32 * |
| Polyphenols | Solubility, mg/mL | logP | logD7.4 |
|---|---|---|---|
| DfTf | more than 12 mg/mL | 1.13 ± 0.12 [31] | - |
| taxifolin | 0.87 [73] 1.0 [74] | 1.57 ± 0.03 [52] | 0.96 ± 0.02 [52] |
| quercetin | 0.060 [75] | 1.82 ± 0.32 [76] 1.26 1 [72] | 0.76 ± 0.09 [77] |
| catechin | 4.54 [78] | 1.31 1 [72] | - |
| hesperetin | 0.001 [79] | 2.6 [80] | - |
| naringenin | 0.00438 [81] | 2.30 ± 0.18 [52] 2.60 ± 0.03 [76] 1.60 1 [72] 2.52 [80] | 2.18 ± 0.06 [52] 2.09 ± 0.14 [82] |
| eriodictyol | 0.070 [75] | 2.27 ± 0.02 [76] 2.02 [80] | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shubina, V.S.; Kozina, V.I.; Shatalin, Y.V. Comparison of Antioxidant Properties of a Conjugate of Taxifolin with Glyoxylic Acid and Selected Flavonoids. Antioxidants 2021, 10, 1262. https://doi.org/10.3390/antiox10081262
Shubina VS, Kozina VI, Shatalin YV. Comparison of Antioxidant Properties of a Conjugate of Taxifolin with Glyoxylic Acid and Selected Flavonoids. Antioxidants. 2021; 10(8):1262. https://doi.org/10.3390/antiox10081262
Chicago/Turabian StyleShubina, Victoria S., Victoria I. Kozina, and Yuri V. Shatalin. 2021. "Comparison of Antioxidant Properties of a Conjugate of Taxifolin with Glyoxylic Acid and Selected Flavonoids" Antioxidants 10, no. 8: 1262. https://doi.org/10.3390/antiox10081262