Inhibitory Effect of Olive Phenolic Compounds Isolated from Olive Oil By-Product on Melanosis of Shrimps
Abstract
:1. Introduction
2. Materials and Methods
2.1. Shrimp Collection
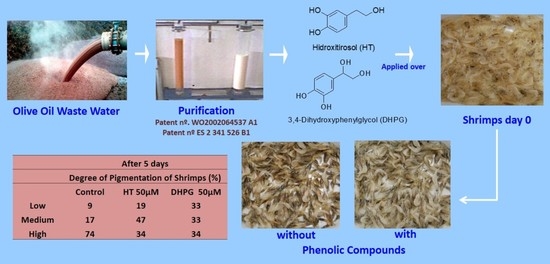
2.2. Isolation of HT and DHPG from Olive Oil By-Product
2.3. Preparation of Novel Phenolic Derivatives Containing Selenium and Sulphur
2.4. Treatment of the Shrimp
2.5. Treatments with HT and DHPG
2.6. Treatments with Diselenide of bis-HT
2.7. Treatments with Selenourea and Thiourea
2.8. Determination of Melanosis Formation in Atlantic Ditch Shrimp
2.9. Inhibition of Tyrosinase Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effects of HT and DHPG on Melanosis Formation of Atlantic Ditch Shrimp
3.2. Effect of Diselenide of bis-HT on Melanosis Formation of Atlantic Ditch Shrimp
3.3. Effects of Selenourea and Thiourea on Melanosis Formation of Atlantic Ditch Shrimp
3.4. Inhibition of Tyrosinase Activity
3.5. Use of Atlantic Ditch Shrimp as Crustaceans Model System for Preliminary Studies on Melanosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palma, J.; Andrade, J.P.; Lemme, A.; Bureau, D.P. Quantitative dietary requirement of juvenile Atlantic ditch shrimp Palae-monetes varians for lysine, methionine and arginine. Aquac. Res. 2015, 46, 1822–1830. [Google Scholar] [CrossRef]
- Wilcox, D.E.; Porras, A.G.; Hwang, Y.T.; Lerch, K.; Winkler, M.E.; Solomon, E.I. Substrate-analog binding to the coupled bi-nuclear copper active-site in tyrosinase. J. Am. Chem. Soc. 1985, 107, 4015–4027. [Google Scholar] [CrossRef]
- Gokoglu, N.; Yerlikaya, P. Inhibition effects of grape seed extracts on melanosis formation in shrimp (Parapenaeus longirostris). Int. J. Food Sci. Tech. 2008, 43, 1004–1008. [Google Scholar] [CrossRef]
- López-Caballero, M.E.; Martínez-Alvarez, O.; Gómez-Guillén, M.D.C.; Montero, P. Quality of thawed deepwater pink shrimp (Parapenaeus longirostris) treated with melanosis-inhibiting formulations during chilled storage. Int. J. Food Sci. Technol. 2007, 42, 1029–1038. [Google Scholar] [CrossRef] [Green Version]
- Montero, P.; Caballero, M.E.L.; Mateos, M.P. The Effect of Inhibitors and High Pressure Treatment to Prevent Melanosis and Microbial Growth on Chilled Prawns (Penaeus japonicus). J. Food Sci. 2008, 66, 1201–1206. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Alvarez, O.; Gómez-Guillén, C.; Montero, P. Effect of different chemical compounds as coadjutants of 4-hexylresorcinol on the appearance of deepwater pink shrimp (Parapenaeus longirostris) during chilled storage. Int. J. Food Sci. Technol. 2008, 43, 2010–2018. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.S.; Wei, C.-I.; Rolle, R.S.; Otwell, W.S.; Balaban, M.O.; Marshall, M.R. Inhibitory effect of kojic acid on some plant and crustacean polyphenol oxidases. J. Agric. Food Chem. 1991, 39, 1396–1401. [Google Scholar] [CrossRef]
- Taoukis, P.S.; Labuza, T.P.; Lillemo, J.H.; Lin, S.W. Inhibition of shrimp melanosis (black spot) by ficin. Lebensm. Wiss. Technol. 1990, 23, 52–54. [Google Scholar]
- Kubo, I.; Chen, Q.-X.; Nihei, K.-I. Molecular design of antibrowning agents: Antioxidative tyrosinase inhibitors. Food Chem. 2003, 81, 241–247. [Google Scholar] [CrossRef]
- Xu, L.-L.; Hu, P.-P.; Kong, X.-L.; Hider, R.C.; Zhou, T.; Dai, Z.-Y. 3-Hydroxypyridinone-l-phenylalanine conjugates with antimicrobial and tyrosinase inhibitory activities as potential shrimp preservatives. Int. J. Food Sci. Technol. 2013, 49, 797–803. [Google Scholar] [CrossRef]
- Mu, H.; Chen, H.; Fang, X.; Mao, J.; Gao, H. Effect of cinnamaldehyde on melanosis and spoilage of Pacific white shrimp (Litopenaeus vannamei) during storage. J. Sci. Food Agric. 2012, 92, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Visessanguan, W.; Tanaka, M. Inhibitory effect of cysteine and glutathione on phenoloxidase from kuruma prawn (Penaeus japonicus). Food Chem. 2006, 98, 158–163. [Google Scholar] [CrossRef]
- Bono, G.; Okpala, C.O.R.; Alberio, G.R.; Messina, C.M.; Santulli, A.; Giacalone, G.; Spagna, G. Toward shrimp consumption without chemicals: Combined effects of freezing and modified atmosphere packaging (MAP) on some quality characteristics of Giant Red Shrimp (Aristaeomorpha foliacea) during storage. Food Chem. 2016, 197, 581–588. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Melanosis and quality changes of Pacific white shrimp (Litopenaeus vannamei) treated with cat-echin during iced storage. J. Agric. Food Chem. 2009, 57, 3578–3586. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Benjakul, S. Effect of ferulic acid on inhibition of polyphenoloxidase and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chem. 2009, 116, 323–331. [Google Scholar] [CrossRef]
- Basiri, S.; Shekarforoush, S.S.; Aminlari, M.; Akbari, S. The effect of pomegranate peel extract (PPE) on the polyphenol oxidase (PPO) and quality of Pacific white shrimp (Litopenaeus vannamei) during refrigerated storage. LWT 2015, 60, 1025–1033. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Use of tea extracts for inhibition of polyphenoloxidase and retardation of quality loss of Pacific white shrimp during iced storage. LWT 2011, 44, 924–932. [Google Scholar] [CrossRef]
- Medina, E.; De Castro, A.; Romero, C.; Brenes, M. Comparison of the Concentrations of Phenolic Compounds in Olive Oils and Other Plant Oils: Correlation with Antimicrobial Activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef]
- Muñoz, A.L.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Palacios-Díaz, R.; Fernández-Bolaños, J. A study of the precursors of the natural antioxidant phenol 3,4-dihydroxyphenylglycol in olive oil waste. Food Chem. 2013, 140, 154–160. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Heredia, A.; Guillén, A.R.; Jimenez-Araujo, A. Production in Large Quantities of Highly Purified Hydroxytyrosol from Liquid−Solid Waste of Two-Phase Olive Oil Processing or “Alperujo”. J. Agric. Food Chem. 2002, 50, 6804–6811. [Google Scholar] [CrossRef] [PubMed]
- Auñon-Calles, D.; Giordano, E.; Bohnenberger, S.; Visioli, F. Hydroxytyrosol is not genotoxic in vitro. Pharmacol. Res. 2013, 74, 87–93. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to polyphenols in olive and maintenance of normal blood HDL cholesterol concentrations (ID 1639, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2848. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.J.; Koketsu, M.; Ishihara, H.; Lee, S.M.; Ha, S.K.; Lee, K.H.; Kang, T.H.; Kima, S.Y. Regulation of Melanin Synthesis by Selenium-Containing Carbohydrates. Chem. Pharm. Bull. 2006, 54, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, S.K.; Koketsu, M.; Lee, K.; Choi, S.Y.; Park, J.-H.; Ishihara, H.; Kim, S.Y. Inhibition of Tyrosinase Activity by N,N-Unsubstituted Selenourea Derivatives. Biol. Pharm. Bull. 2005, 28, 838–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.H.; Lim, Y.-J.; Ha, S.K.; Kang, T.H.; Koketsu, M.; Kang, C.; Kim, S.Y.; Park, J.-H. Inhibitory effects of 5-chloroacetyl-2-piperidino-1,3-selenazole, a novel selenium-containing compound, on skin melanin biosynthesis. J. Pharm. Pharmacol. 2010, 62, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J.M.; Maya-Castilla, I.; Rubio-Senent, F.; López, O.; Marset, A. Method for Obtaining Hydroxytyrosol Extract, Mixture of Hydroxytyrosol and 3,4-Dihydroxyphenylglycol Extract, and Hydroxytyrosyl Acetate Extract from By-Products of the Olive Tree and the Purification of Thereof. International Patent WO2013007850A, 17 January 2013. [Google Scholar]
- Pluempanupat, W.; Chantarasriwong, O.; Taboonpong, P.; Jang, D.O.; Chavasiri, W. Reactivity of chlorinating agents/PPh3 for the chlorination of alcohols and carboxylic acids: A comparative study. Tetrahedron Lett. 2007, 48, 223–226. [Google Scholar] [CrossRef]
- Klayman, D.L.; Griffin, T.S. Reaction of selenium with sodium borohydride in protic solvents. A Facile Method for the in-troduction of selenium into organic molecules. J. Am. Chem. Soc. 1973, 95, 197–199. [Google Scholar] [CrossRef]
- Lopez, O.; Maya, I.; Fuentes, J.; Fernández-Bolaños, J.G. Simple and efficient synthesis of O-unprotected glycosyl thiourea and isourea derivatives from glycosylamines. Tetrahedron 2004, 60, 61–72. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Shi, J.; Yu, C.; Zhao, M.; Xue, S.; Jiang, Y. Enhanced antioxidant and antityrosinase activities of longan fruit pericarp by ultra-high-pressure-assisted extraction. J. Pharm. Biomed. Anal. 2010, 51, 471–477. [Google Scholar] [CrossRef]
- Baek, S.; Kim, J.; Kim, D.; Lee, C.; Kim, J.; Chung, D.K.; Lee, C. Inhibitory effect of dalbergioidin isolated from the trunk of Lespedeza cyrtobotrya on melanin biosynthesis. J. Microbiol. Biotechn. 2008, 18, 874–879. [Google Scholar]
- Rodríguez, G.; Rodríguez, R.; Fernández-Bolaños, J.; Guillén, R.; Jiménez, A. Antioxidant activity of effluent during the pu-rification of hydroxytyrosol and 3,4-dihydroxyphenylglycol from olive oil waste. Eur. Food Res. Technol. 2007, 224, 733–741. [Google Scholar] [CrossRef]
- Rodríguez, G.; Lama, A.; Trujillo, M.; Espartero, J.L.; Fernández-Bolaños, J. Isolation of a powerful antioxidant from Olea europaea fruit-mill waste: 3,4-Dihydroxyphenylglycol. LWT 2009, 42, 483–490. [Google Scholar] [CrossRef]
- Vogna, D.; Pezzella, A.; Panzella, L.; Napolitano, A.; D’Ischia, M. Oxidative chemistry of hydroxytyrosol: Isolation and characterisation of novel methanooxocinobenzodioxinone derivatives. Tetrahedron Lett. 2003, 44, 8289–8292. [Google Scholar] [CrossRef]
- Bono, G.; Badalucco, C.; Corrao, A.; Cusumano, S.; Mammina, L.; Palmegiano, G.B. Effect of temporal variation, gender and size on cuticle polyphenol oxidase activity in deep-water rose shrimp (Parapenaeus longirostris). Food Chem. 2010, 123, 489–493. [Google Scholar] [CrossRef]



| Degree of Pigmentation (%) | ||||||
|---|---|---|---|---|---|---|
| Treatment | Low | Medium | High | Total of Shrimps | ||
| Exp. 1 6 days | Control | 25 | 10 | 65 a | 40 | |
| HT | 0.1 mM | 19 | 51 | 30 b | 37 | |
| 0.5 | 0 | 87 | 13 bc | 53 | ||
| 1.0 | 0 | 82 | 18 bc | 68 | ||
| 5.0 | 0 | 70 | 30 a | 50 | ||
| DHPG | 0.1 mM | 76 | 24 | 0 e | 50 | |
| 0.5 | 75 | 25 | 0 de | 43 | ||
| 1.0 | 70 | 26 | 4 d | 51 | ||
| 2.5 | 36 | 22 | 42 b | 40 | ||
| 5.0 | 17 | 48 | 35 b | 46 | ||
| Exp. 2 3 days | Control | 15 | 51 | 34 a | 387 | |
| HT | 10 μM | 18 | 57 | 25 ab | 415 | |
| 50 | 24 | 48 | 28 ab | 357 | ||
| 100 | 11 | 71 | 18 bc | 387 | ||
| DHPG | 10 μM | 33 | 48 | 19 c | 346 | |
| 50 | 49 | 42 | 9 d | 341 | ||
| 100 | 17 | 57 | 26 ab | 380 | ||
| 500 | 17 | 65 | 17 bc | 377 | ||
| HT/DHPG | 50 µM each | 15 | 68 | 17 bc | 323 | |
| Exp. 3 5 days | Control | 5 | 27 | 68 a | 332 | |
| HT | 10 µM | 16 | 42 | 41 c | 403 | |
| 25 | 14 | 34 | 52 bc | 352 | ||
| 50 | 18 | 38 | 44 c | 215 | ||
| 75 | 14 | 40 | 46 c | 301 | ||
| DHPG | 10 µM | 23 | 35 | 42 c | 317 | |
| 25 | 16 | 19 | 65 ab | 310 | ||
| 50 | 17 | 56 | 27 d | 253 | ||
| 75 | 16 | 39 | 44 bc | 295 | ||
| AA/AC | 50 μM each | 17 | 23 | 60 bc | 287 | |
| Treatment | Degree of Pigmentation (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 Days | 5 Days | Total of Shrimps | ||||||
| Low | Medium | High | Low | Medium | High | |||
| Control | 36 | 43 | 21 A | 9 | 17 | 74 a | 130 | |
| Diselenide of bis-HT | 0.5 µM | 31 | 44 | 26 AB | 21 | 30 | 49 bc | 119 |
| 1 | 61 | 37 | 2 G | 17 | 13 | 69 ab | 120 | |
| 10 | 52 | 40 | 8 CD | 27 | 27 | 46 cd | 125 | |
| 20 | 67 | 29 | 4 G | 19 | 27 | 54 bc | 124 | |
| HT | 10 µM | 57 | 34 | 8 DEF | 18 | 26 | 56 bc | 132 |
| 50 | 46 | 48 | 6 CD | 19 | 47 | 34 de | 120 | |
| 75 | 32 | 43 | 26 AB | 10 | 28 | 62 ab | 101 | |
| DHPG | 10 µM | 60 | 35 | 4 F | 20 | 16 | 64 ab | 123 |
| 50 | 62 | 31 | 6 EF | 33 | 33 | 34 ef | 116 | |
| 75 | 47 | 39 | 14 C | 24 | 37 | 39 d | 128 | |
| Treatment | Degree of Pigmentation (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 Days | 5 Days | Total of Shrimps | ||||||
| Low | Medium | High | Low | Medium | High | |||
| Control | 41 | 55 | 5 A | 14 | 34 | 52 a | 165 | |
| Selenourea | 1 µM | 48 | 46 | 6 B | 14 | 43 | 44 ab | 177 |
| 10 | 83 | 15 | 2 F | 13 | 43 | 44 ab | 156 | |
| 20 | 71 | 27 | 2 EF | 16 | 45 | 39 bc | 176 | |
| Thiourea | 1 µM | 49 | 41 | 10 A | 18 | 32 | 50 a | 172 |
| 10 | 43 | 51 | 6 A | 8 | 50 | 42 ab | 159 | |
| 20 | 56 | 40 | 4 CD | 13 | 52 | 34 bc | 173 | |
| DHPG | 20 µM | 57 | 39 | 4 CD | 13 | 50 | 37 bc | 162 |
| 50 | 65 | 29 | 5 D | 32 | 34 | 34 c | 162 | |
| 75 | 45 | 51 | 4 BC | 22 | 32 | 46 ab | 164 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lama-Muñoz, A.; Gómez-Carretero, A.; Rubio-Senent, F.; Bermúdez-Oria, A.; Maya, I.; Fernández-Bolaños, J.G.; Vioque, B.; Fernández-Bolaños, J. Inhibitory Effect of Olive Phenolic Compounds Isolated from Olive Oil By-Product on Melanosis of Shrimps. Antioxidants 2021, 10, 728. https://doi.org/10.3390/antiox10050728
Lama-Muñoz A, Gómez-Carretero A, Rubio-Senent F, Bermúdez-Oria A, Maya I, Fernández-Bolaños JG, Vioque B, Fernández-Bolaños J. Inhibitory Effect of Olive Phenolic Compounds Isolated from Olive Oil By-Product on Melanosis of Shrimps. Antioxidants. 2021; 10(5):728. https://doi.org/10.3390/antiox10050728
Chicago/Turabian StyleLama-Muñoz, Antonio, Antonio Gómez-Carretero, Fátima Rubio-Senent, Alejandra Bermúdez-Oria, Inés Maya, José G. Fernández-Bolaños, Blanca Vioque, and Juan Fernández-Bolaños. 2021. "Inhibitory Effect of Olive Phenolic Compounds Isolated from Olive Oil By-Product on Melanosis of Shrimps" Antioxidants 10, no. 5: 728. https://doi.org/10.3390/antiox10050728