Protective Effect of Oligonol on Dimethylnitrosamine-Induced Liver Fibrosis in Rats via the JNK/NF-κB and PI3K/Akt/Nrf2 Signaling Pathways
Abstract
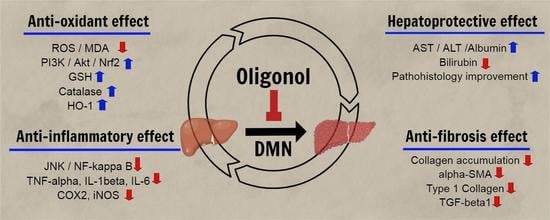
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Induction of Liver Fibrosis with DMN
2.3. Histology
2.4. Biochemical Analysis of Serum Parameters
2.5. Malondialdehyde (MDA) Content
2.6. Measurement of ROS Level
2.7. Determination of Total Protein SH, Non-Protein SH Level, and Catalase Activity
2.8. RNA Extraction and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
2.9. Western Blotting Analysis
2.10. Immunohistochemistry
2.11. Effect of Oligonol on HSC Proliferation
2.12. Statistical Analyses
3. Results
3.1. Changes in Serum Parameters in Rats Intoxicated by DMN
3.2. Liver Histopathology
3.3. Suppression of the DMN-Induced Oxidative Stress by Oligonol Treatment
3.4. Effects of Oligonol against DMN-Induced Liver Inflammation and NF-κB Activation
3.5. Effects of Oligonol on Nrf2 Activation and Akt Phosphorylation in DMN-Induced Liver Injuries
3.6. Antifibrotic Effect of Oligonol in DMN-Induced Chronic Liver Injuries
3.7. The Effects of Oligonol on HSC-T6 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melgert, B.N.; Olinga, P.; Van Der Laan, J.M.; Weert, B.; Cho, J.; Schuppan, D.; Groothuis, G.M.; Meijer, D.K.; Poelstra, K. Targeting dexamethasone to Kupffer cells: Effects on liver inflammation and fibrosis in rats. Hepatology 2001, 34, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tovar, E.; Muriel, P. Molecular mechanisms that link oxidative stress, inflammation, and fibrosis in the liver. Antioxidants 2020, 9, 1279. [Google Scholar] [CrossRef]
- Manibusan, M.K.; Odin, M.; Eastmond, D.A. Postulated carbon tetrachloride mode of action: A review. J. Environ. Sci. Health Part C 2007, 25, 185–209. [Google Scholar] [CrossRef]
- Muriel, P. Peroxidation of lipids and liver damage. In Antioxidants, Oxidants, and Free Radicals, 1st ed.; Baskin, S.I., Salem, H., Eds.; CRC Press: Boca Raton, FL, USA, 1997; pp. 237–257. [Google Scholar] [CrossRef]
- Shin, D.-S.; Kim, K.W.; Chung, H.Y.; Yoon, S.; Moon, J.-O. Effect of sinapic acid against carbon tetrachloride-induced acute hepatic injury in rats. Arch. Pharm. Res. 2013, 36, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.-S.; Kim, K.W.; Chung, H.Y.; Yoon, S.; Moon, J.-O. Effect of sinapic acid against dimethylnitrosamine-induced hepatic fibrosis in rats. Arch. Pharm. Res. 2013, 36, 608–618. [Google Scholar] [CrossRef]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.S.; Park, C.H.; Yokozawa, T. Treatment with oligonol, a low-molecular polyphenol derived from lychee fruit, attenuates diabetes-induced hepatic damage through regulation of oxidative stress and lipid metabolism. Br. J. Nutr. 2011, 106, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Noh, J.S.; Fujii, H.; Roh, S.-S.; Song, Y.-O.; Choi, J.S.; Chung, H.Y.; Yokozawa, T. Oligonol, a low-molecular-weight polyphenol derived from lychee fruit, attenuates gluco-lipotoxicity-mediated renal disorder in type 2 diabetic db/db mice. Drug Discov. Ther. 2015, 9, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Xu, B.; Nie, L.; He, K.; Zhou, L.; Huang, X.; Spencer, P.; Yang, X.; Liu, J. Flavanol-rich lychee fruit extract substantially reduces progressive cognitive and molecular deficits in a triple-transgenic animal model of alzheimer disease. Nutr. Neurosci. 2019, 1–15. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, J.Y.; Kim, M.Y.; Shin, S.H.; Roh, S.-S.; Choi, J.S.; Chung, H.Y.; Song, Y.-O.; Shin, Y.S.; Yokozawa, T. Oligonol, a low-molecular-weight polyphenol derived from lychee fruit, protects the pancreas from apoptosis and proliferation via oxidative stress in streptozotocin-induced diabetic rats. Food Funct. 2016, 7, 3056–3063. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-W.; Chen, Y.-J.; Chang, Y.-C.; Chang, S.-J. Oligonol, a low-molecular weight polyphenol derived from lychee, alleviates muscle loss in diabetes by suppressing Atrogin-1 and MuRF1. Nutrients 2017, 9, 1040. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Park, J.-M.; Lee, J.-S.; Kim, Y.; Kangwan, N.; Han, Y.-M.; Kang, E.; An, J.; Park, Y.; Hahm, K.-B. Oligonol prevented the relapse of dextran sulfate sodium-ulcerative colitis through enhancing NRF2-mediated antioxidative defense mechanism. J. Physiol. Pharmacol. 2018, 69. [Google Scholar] [CrossRef]
- Bak, J.; Je, N.K.; Chung, H.Y.; Yokozawa, T.; Yoon, S.; Moon, J.-O. Oligonol ameliorates CCl4-induced liver injury in rats via the NF-Kappa B and MAPK signaling pathways. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Cheong, K.O.; Shin, D.-S.; Bak, J.; Lee, C.; Kim, K.W.; Je, N.K.; Chung, H.Y.; Yoon, S.; Moon, J.-O. Hepatoprotective effects of zingerone on carbon tetrachloride-and dimethylnitrosamine-induced liver injuries in rats. Arch. Pharm. Res. 2016, 39, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Chase, S.D. Rapid, fluorimetric–liquid chromatographic determination of malondialdehyde in biological samples. J. Chromatogr. B 2002, 775, 121–126. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′, 7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Higach, T. Critical review on the determination of glutathione in biological preparation. Tanpakushitsu Kakusan Koso 1988, 33, 1370. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Dicker, E.; Cederbaum, A.I. Increased oxidation of dimethylnitrosamine in pericentral microsomes after pyrazole induction of cytochrome P-4502El. Alcohol. Clin. Exp. Res. 1991, 15, 1072–1076. [Google Scholar] [CrossRef]
- Jezequel, A.; Mancini, R.; Rinaldesi, M.; Ballardini, G.; Fallani, M.; Bianchi, F.; Orlandi, F. Dimethylnitrosamine-induced cirrhosis: Evidence for an immunological mechanism. J. Hepatol. 1989, 8, 42–52. [Google Scholar] [CrossRef]
- Muriel, P. Hepatotoxicity: From Genomics to In Vitro and In Vivo Models; John Wiley and Sons LTD.: West Sussex, UK, 2007; pp. 371–389. ISBN 978-047-005-716-2. [Google Scholar]
- Muriel, P. NF-κB in liver diseases: A target for drug therapy. J. Appl. Toxicol. 2009, 29, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.; Macia, M.G.; Luli, S.; Worrell, J.C.; Reilly, W.J.; Paish, H.L.; Knox, A.; Barksby, B.S.; Gee, L.M.; Zaki, M.Y.W.; et al. c-Rel orchestrates energy-dependent epithelial and macrophage reprogramming in fibrosis. Nat. Metab. 2020, 2, 1350–1367. [Google Scholar] [CrossRef]
- Fullard, N.; Wilson, C.L.; Oakley, F. Roles of c-Rel signalling in inflammation and disease. Int. J. Biochem. Cell Biol. 2012, 44, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.-J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef]
- George, J.; Tsuchishima, M.; Tsutsumi, M. Molecular mechanisms in the pathogenesis of N-nitrosodimethylamine induced hepatic fibrosis. Cell Death Dis. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Jaiswal, A.K. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radic. Biol. Med. 2013, 57, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Enomoto, A.; Itoh, K.; Nagayoshi, E.; Haruta, J.; Kimura, T.; O’Connor, T.; Harada, T.; Yamamoto, M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001, 59, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messier, E.M.; Bahmed, K.; Tuder, R.M.; Chu, H.W.; Bowler, R.P.; Kosmider, B. Trolox contributes to Nrf2-mediated protection of human and murine primary alveolar type II cells from injury by cigarette smoke. Cell Death Dis. 2013, 4, e573. [Google Scholar] [CrossRef] [PubMed]
- Iranshahy, M.; Iranshahi, M.; Abtahi, S.R.; Karimi, G. The role of nuclear factor erythroid 2-related factor 2 in hepatoprotective activity of natural products: A review. Food Chem. Toxicol. 2018, 120, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, S.; Ishida, K.; Kameyama, N.; Ueyama, J.; Hattori, A.; Tatsumi, Y.; Hayashi, H.; Yano, M.; Hayashi, K.; Katano, Y. Ursodeoxycholic acid induces glutathione synthesis through activation of PI3K/Akt pathway in HepG2 cells. Biochem. Pharmacol. 2009, 77, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Odagiri, N.; Matsubara, T.; Sato-Matsubara, M.; Fujii, H.; Enomoto, M.; Kawada, N. Anti-fibrotic treatments for chronic liver diseases: The present and the future. Clin. Mol. Hepatol. 2020. [Google Scholar] [CrossRef]










| Gene | Sequences | Gene Access Number | |
|---|---|---|---|
| TNF-α | F | TTCTGTCTACTGAACTTGGGGGTGATCGGTCC | XM_032888689.1 |
| R | GTATGAGATAGCAAATCGGCTGACGGTGTGGG | ||
| IL-1β | F | ATGGCAACTGTTCCTGAACTCAACT | NM_031512.2 |
| R | CAGGACAGGTATAGATTCTTTCCTTT | ||
| IL-6 | F | CGAGCCCACCAGGAACGAAAGTC | M26745.1 |
| R | CTGGCTGGAAGTCTCTTGCGGAG | ||
| iNOS | F | GATTCAGTGGTCCAACCTGCA | L12562.1 |
| R | CGACCTGATGTTGCCACTGTT | ||
| COX-2 | F | CCAGAGCAGAGAGATGAAATACCA | NM_017232.3 |
| R | GCAGGGCGGGATACAGTTC | ||
| TGF-β1 | F | TATAGCAACAATTCCTGGCG | NM_021578.2 |
| R | TGCTGTCACAGGAGCAGTG | ||
| α-SMA | F | CCGAGATCTCACCGACTACC | XM_032891814.1 |
| R | TCCAGAGCGACATAGCACAG | ||
| COL1 | F | AAGAAGGCGGCAAAGGTC | XM_005257059.4 |
| R | GGACCTTGTTTGCCAGGT | ||
| GAPDH | F | GACAACTTTGGCATCGTGGA | NM_017008.4 |
| R | ATGCAGGGATGATGTTCTGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Bak, J.; Yoon, S.; Moon, J.-O. Protective Effect of Oligonol on Dimethylnitrosamine-Induced Liver Fibrosis in Rats via the JNK/NF-κB and PI3K/Akt/Nrf2 Signaling Pathways. Antioxidants 2021, 10, 366. https://doi.org/10.3390/antiox10030366
Lee C, Bak J, Yoon S, Moon J-O. Protective Effect of Oligonol on Dimethylnitrosamine-Induced Liver Fibrosis in Rats via the JNK/NF-κB and PI3K/Akt/Nrf2 Signaling Pathways. Antioxidants. 2021; 10(3):366. https://doi.org/10.3390/antiox10030366
Chicago/Turabian StyleLee, Changyong, Jeonghyeon Bak, Sik Yoon, and Jeon-Ok Moon. 2021. "Protective Effect of Oligonol on Dimethylnitrosamine-Induced Liver Fibrosis in Rats via the JNK/NF-κB and PI3K/Akt/Nrf2 Signaling Pathways" Antioxidants 10, no. 3: 366. https://doi.org/10.3390/antiox10030366