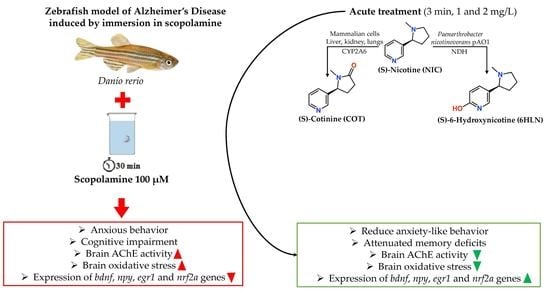
Anxiolytic, Promnesic, Anti-Acetylcholinesterase and Antioxidant Effects of Cotinine and 6-Hydroxy-L-Nicotine in Scopolamine-Induced Zebrafish (Danio rerio) Model of Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Chemicals and Reagents
2.3. Treatment and Group Division
2.4. Behavioral Tasks
2.4.1. Novel Tank Diving Test
2.4.2. Y-Maze Test
2.4.3. Object Discrimination Test
2.5. Biochemical Parameter Analysis
2.5.1. Protein Concentration Determination
2.5.2. Evaluation of AChE Activity
2.5.3. Evaluation of SOD Activity
2.5.4. Evaluation of CAT Activity
2.5.5. Evaluation of GPX Activity
2.5.6. GSH Content
2.5.7. Carbonylated Proteins Levels
2.5.8. MDA Levels
2.6. RNA Purification and Real-Time Quantitative PCR (RT-qPCR) Analysis
2.7. Statistic Interpretation
3. Results and Discussion
3.1. The Effects of Cotinine and 6-Hydroxy-L-Nicotine on Anxiety
3.2. The Effects of Cotinine and 6-Hydroxy-L-Nicotine on Cognition
3.3. The Effects of Cotinine and 6-Hydroxy-L-Nicotine on Acetylcholinesterase Activity
3.4. The Effects of Cotinine and 6-Hydroxy-L-Nicotine on Oxidative Stress
3.5. The Effects of Cotinine and 6-Hydroxy-L-Nicotine on Gene Expression
3.5.1. Brain-Derived Neurotrophic Factor (BDNF) Expression
3.5.2. Neuropeptide Y (NPY) Expression
3.5.3. Early Growth Response Protein 1 (Egr1) Expression
3.5.4. Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2a) Expression
3.6. Pearson Correlations between Behavioral, Biochemical, and Genetic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodson, R. Alzheimer’s disease. Nature 2018, 559, S1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.; Stomrud, E.; Lindberg, O.; Westman, E.; Johansson, P.M.; van Westen, D.; Mattsson, N.; Hansson, O. Apathy and anxiety are early markers of Alzheimer’s disease. Neurobiol. Aging 2020, 85, 74–82. [Google Scholar] [CrossRef]
- Calderon-Garcidueñas, A.L.; Duyckaerts, C. Alzheimer disease. In Handbook of Clinical Neurology; Elsevier: New York, NY, USA, 2018; Volume 145, pp. 325–337. [Google Scholar]
- Takahashi, R.H.; Nagao, T.; Gouras, G.K. Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer’s disease. Pathol. Int. 2017, 67, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Maskos, U. Role of the nicotinic acetylcholine receptor in Alzheimer’s disease pathology and treatment. Neuropharmacology 2015, 96, 255–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, M.; Ebrahimie, E.; Lardelli, M. Using the zebrafish model for Alzheimer’s disease research. Front. Genet. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Kannan, R.R. Zebrafish: An emerging real-time model system to study Alzheimer’s disease and neurospecific drug discovery. Cell Death Discov. 2018, 4, 45. [Google Scholar] [CrossRef] [Green Version]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norton, W.; Bally-Cuif, L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010, 11, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deiana, S.; Harrington, C.R.; Wischik, C.M.; Riedel, G. Methylthioninium chloride reverses cognitive deficits induced by scopolamine: Comparison with rivastigmine. Psychopharmacology 2009, 202, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L.S. Theory-Driven Approaches to Cognitive Enhancement; Colzato, L.S., Ed.; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-57504-9. [Google Scholar]
- Sofuoglu, M.; Herman, A.I.; Robinson, C.; Waters, A.J. Cognitive Effects of Nicotine. In The Effects of Drug Abuse on the Human Nervous System; Elsevier: New York, NY, USA, 2014; pp. 367–385. ISBN 9780124186798. [Google Scholar]
- Guan, Z.-Z.; Yu, W.-F.; Nordberg, A. Dual effects of nicotine on oxidative stress and neuroprotection in PC12 cells. Neurochem. Int. 2003, 43, 243–249. [Google Scholar] [CrossRef]
- Nizri, E.; Irony-Tur-Sinai, M.; Lory, O.; Orr-Urtreger, A.; Lavi, E.; Brenner, T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J. Immunol. 2009, 183, 6681–6688. [Google Scholar] [CrossRef] [Green Version]
- Nordberg, A.; Hellström-Lindahl, E.; Lee, M.; Johnson, M.; Mousavi, M.; Hall, R.; Perry, E.; Bednar, I.; Court, J. Chronic nicotine treatment reduces beta-amyloidosis in the brain of a mouse model of Alzheimer’s disease (APPsw). J. Neurochem. 2002, 81, 655–658. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Fraiman, J.B. Cardiovascular effects of electronic cigarettes. Nat. Rev. Cardiol. 2017, 14, 447–456. [Google Scholar] [CrossRef]
- Carr, E. Targeting Nicotine Addiction. Clin. J. Oncol. Nurs. 2018, 22, 243–244. [Google Scholar] [CrossRef]
- Buccafusco, J.J. Neuronal nicotinic receptor subtypes: Defining therapeutic targets. Mol. Interv. 2004, 4, 285–295. [Google Scholar] [CrossRef]
- Pogocki, D.; Ruman, T.; Danilczuk, M.; Danilczuk, M.; Celuch, M.; Wałajtys-Rode, E. Application of nicotine enantiomers, derivatives and analogues in therapy of neurodegenerative disorders. Eur. J. Pharmacol. 2007, 563, 18–39. [Google Scholar] [CrossRef]
- Echeverria, V.; Zeitlin, R. Cotinine: A Potential New Therapeutic Agent against Alzheimer’s disease. CNS Neurosci. Ther. 2012, 18, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Grizzell, J.A.; Echeverria, V. New Insights into the Mechanisms of Action of Cotinine and its Distinctive Effects from Nicotine. Neurochem. Res. 2015, 40, 2032–2046. [Google Scholar] [CrossRef] [PubMed]
- Moran, V.E. Cotinine: Beyond that Expected, More than a Biomarker of Tobacco Consumption. Front. Pharmacol. 2012, 3, 173. [Google Scholar] [CrossRef] [Green Version]
- Igloi, G.L.; Brandsch, R. Sequence of the 165-Kilobase Catabolic Plasmid pAO1 from Arthrobacter nicotinovorans and Identification of a pAO1-Dependent Nicotine Uptake System. J. Bacteriol. 2003, 185, 1976–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandsch, R. Microbiology and biochemistry of nicotine degradation. Appl. Microbiol. Biotechnol. 2006, 69, 493–498. [Google Scholar] [CrossRef]
- Boiangiu, R.; Guzun, D.; Mihăşan, M. Time dependent accumulation of nicotine derivatives in the culture medium of Arthrobacter nicotinovorans pAO1. Analele Stiint. ale Univ. “Alexandru Ioan Cuza” din Iasi Sec. II a. Genet. si Biol. Mol. 2014, 15, 19–23. [Google Scholar]
- Hritcu, L.; Mihasan, M. 6-Hydroxy-l-Nicotine and Memory Impairment. In Neuroscience of Nicotine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–172. [Google Scholar]
- Hritcu, L.; Ionita, R.; Motei, D.E.; Babii, C.; Stefan, M.; Mihasan, M. Nicotine versus 6-hydroxy-l-nicotine against chlorisondamine induced memory impairment and oxidative stress in the rat hippocampus. Biomed. Pharmacother. 2017, 86, 102–108. [Google Scholar] [CrossRef]
- Hritcu, L.; Stefan, M.; Brandsch, R.; Mihasan, M. Enhanced behavioral response by decreasing brain oxidative stress to 6-hydroxy-l-nicotine in Alzheimer’s disease rat model. Neurosci. Lett. 2015, 591, 41–47. [Google Scholar] [CrossRef]
- Boiangiu, R.S.; Mihasan, M.; Gorgan, D.L.; Stache, B.A.; Petre, B.A.; Hritcu, L. Cotinine and 6-Hydroxy-L-Nicotine Reverses Memory Deficits and Reduces Oxidative Stress in Aβ25-35-Induced Rat Model of Alzheimer’s Disease. Antioxidants 2020, 9, 768. [Google Scholar] [CrossRef]
- Patel, S.; Grizzell, J.A.; Holmes, R.; Zeitlin, R.; Solomon, R.; Sutton, T.L.; Rohani, A.; Charry, L.C.; Iarkov, A.; Mori, T.; et al. Cotinine halts the advance of Alzheimer’s disease-like pathology and associated depressive-like behavior in Tg6799 mice. Front. Aging Neurosci. 2014, 6, 162. [Google Scholar] [CrossRef]
- Echeverria, V.; Zeitlin, R.; Burgess, S.; Patel, S.; Barman, A.; Thakur, G.; Mamcarz, M.; Wang, L.; Sattelle, D.B.; Kirschner, D.A.; et al. Cotinine reduces amyloid-β aggregation and improves memory in Alzheimer’s disease mice. J. Alzheimer’s Dis. 2011, 24, 817–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinza, I.; Abd-Alkhalek, A.M.; El-Raey, M.A.; Boiangiu, R.S.; Eldahshan, O.A.; Hritcu, L. Ameliorative effects of rhoifolin in scopolamine-induced amnesic zebrafish (Danio rerio) model. Antioxidants 2020, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, G.; El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Boiangiu, R.S.; Todirascu-Ciornea, E.; Hritcu, L.; Singab, A.N.B. Agathisflavone isolated from Schinus polygamus (Cav.) Cabrera leaves prevents scopolamine-induced memory impairment and brain oxidative stress in zebrafish (Danio rerio). Phytomedicine 2019, 58, 152889. [Google Scholar] [CrossRef] [PubMed]
- Capatina, L.; Boiangiu, R.S.; Dumitru, G.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Todirascu-Ciornea, E. Rosmarinus officinalis Essential Oil Improves Scopolamine-Induced Neurobehavioral Changes via Restoration of Cholinergic Function and Brain Antioxidant Status in Zebrafish (Danio rerio). Antioxidants 2020, 9, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, M.L.; Oreschak, K.; Rhinehart, Z.; Robison, B.D. Anxiolytic effects of fluoxetine and nicotine exposure on exploratory behavior in zebrafish. PeerJ 2016, 4, e2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, T.; Fontana, B.D.; Müller, T.E.; Bertoncello, K.T.; Canzian, J.; Rosemberg, D.B. Nicotine prevents anxiety-like behavioral responses in zebrafish. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 94, 109655. [Google Scholar] [CrossRef] [PubMed]
- Ziani, P.R.; Müller, T.E.; Stefanello, F.V.; Fontana, B.D.; Duarte, T.; Canzian, J.; Rosemberg, D.B. Nicotine increases fear responses and brain acetylcholinesterase activity in a context-dependent manner in zebrafish. Pharmacol. Biochem. Behav. 2018, 170, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Grossman, L.; Collier, A.D.; Echevarria, D.J.; Kalueff, A.V. Anxiogenic-like effects of chronic nicotine exposure in zebrafish. Pharmacol. Biochem. Behav. 2015, 139, 112–120. [Google Scholar] [CrossRef]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C.; et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010, 5, 1786–1799. [Google Scholar] [CrossRef]
- Rosemberg, D.B.; Rico, E.P.; Mussulini, B.H.M.; Piato, Â.L.; Calcagnotto, M.E.; Bonan, C.D.; Dias, R.D.; Blaser, R.E.; Souza, D.O.; de Oliveira, D.L. Differences in Spatio-Temporal Behavior of Zebrafish in the Open Tank Paradigm after a Short-Period Confinement into Dark and Bright Environments. PLoS ONE 2011, 6, e19397. [Google Scholar] [CrossRef] [Green Version]
- Cognato, G.d.P.; Bortolotto, J.W.; Blazina, A.R.; Christoff, R.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Y-Maze memory task in zebrafish (Danio rerio): The role of glutamatergic and cholinergic systems on the acquisition and consolidation periods. Neurobiol. Learn. Mem. 2012, 98, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Zanandrea, R.; Abreu, M.S.; Piato, A.; Barcellos, L.J.G.; Giacomini, A.C.V.V. Lithium prevents scopolamine-induced memory impairment in zebrafish. Neurosci. Lett. 2018, 664, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, F.V.; Fontana, B.D.; Ziani, P.R.; Müller, T.E.; Mezzomo, N.J.; Rosemberg, D.B. Exploring Object Discrimination in Zebrafish: Behavioral Performance and Scopolamine-Induced Cognitive Deficits at Different Retention Intervals. Zebrafish 2019, 16, 370–378. [Google Scholar] [CrossRef]
- Gaspary, K.V.; Reolon, G.K.; Gusso, D.; Bonan, C.D. Novel object recognition and object location tasks in zebrafish: Influence of habituation and NMDA receptor antagonism. Neurobiol. Learn. Mem. 2018, 155, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Faillace, M.; Pisera-Fuster, A.; Medrano, M.; Bejarano, A.; Bernabeu, R. Short- and long-term effects of nicotine and the histone deacetylase inhibitor phenylbutyrate on novel object recognition in zebrafish. Psychopharmacology 2017, 234, 943–955. [Google Scholar] [CrossRef]
- Oliveira, J.; Silveira, M.; Chacon, D.; Luchiari, A. The zebrafish world of colors and shapes: Preference and discrimination. Zebrafish 2015, 12, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Valentim, A.M.; Olsson, I.A.S.; Valentim, A.M.; van Eeden, F.J.; Strähle, U. Euthanizing zebrafish legally in Europe: Are the approved methods of euthanizing zebrafish appropriate to research reality and animal welfare? EMBO Rep. 2016, 17, 1688–1689. [Google Scholar] [CrossRef] [Green Version]
- Gupta, T.; Mullins, M.C. Dissection of Organs from the Adult Zebrafish. J. Vis. Exp. 2010, 37, e1717. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Ellman, G.; Courtney, K.; Andres, V.J.; Feather-Stone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Winterbourn, C.; Hawkins, R.; Brian, M.; Carrell, R. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975, 85, 337. [Google Scholar] [PubMed]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Tokumura, A. Glutathione peroxidase activity in tissues of vitamin E-deficient mice. J. Nutr. Sci. Vitaminol. 1976, 22, 405–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [PubMed]
- Salbitani, G.; Vona, V.; Bottone, C.; Petriccione, M.; Carfagna, S. Sulfur deprivation results in oxidative perturbation in chlorella sorokiniana (211/8k). Plant Cell Physiol. 2015, 56, 897–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-related changes in oxidized proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Luo, S.; Wehr, N.B. Protein carbonylation: Avoiding pitfalls in the 2,4-dinitrophenylhydrazine assay. Redox Rep. 2009, 14, 159–166. [Google Scholar] [CrossRef]
- Domijan, A.-M.; Ralić, J.; Radić Brkanac, S.; Rumora, L.; Žanić-Grubišić, T. Quantification of malondialdehyde by HPLC-FL—Application to various biological samples. Biomed. Chromatogr. 2015, 29, 41–46. [Google Scholar] [CrossRef]
- Vaideș-Negustor, R.N.; Mihăşan, M. Side comparation of two methods for quantifying malondialdehyde levels in animal tissue extracts. J. Exp. Molec. Biol. 2020, 20, 61–66. [Google Scholar]
- Ionita, R.; Postu, P.A.; Mihasan, M.; Gorgan, D.L.; Hancianu, M.; Cioanca, O.; Hritcu, L. Ameliorative effects of Matricaria chamomilla L. hydroalcoholic extract on scopolamine-induced memory impairment in rats: A behavioral and molecular study. Phytomedicine 2018, 47, 113–120. [Google Scholar] [CrossRef]
- Zhao, Q.F.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Xu, W.; Li, J.Q.; Wang, J.; Lai, T.J.; et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J. Affect. Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Zarrindast, M.R.; Khakpai, F. The modulatory role of nicotine on cognitive and non-cognitive functions. Brain Res. 2019, 1710, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.; Stewart, A.; Utterback, E.; Hart, P.; Gaikwad, S.; Wong, K.; Kyzar, E.; Wu, N.; Kalueff, A.V. Three-Dimensional Neurophenotyping of Adult Zebrafish Behavior. PLoS ONE 2011, 6, e17597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, J.; Goebert, D.; Takeshita, J.; Lu, B.Y.; Kang, M. Treatment of Generalized Anxiety Disorder: A Comprehensive Review of the Literature for Psychopharmacologic Alternatives to Newer Antidepressants and Benzodiazepines. Prim. Care Companion CNS Disord. 2011, 13. [Google Scholar] [CrossRef]
- Volgin, A.D.; Yakovlev, O.A.; Demin, K.A.; Alekseeva, P.A.; Kalueff, A.V. Acute behavioral effects of deliriant hallucinogens atropine and scopolamine in adult zebrafish. Behav. Brain Res. 2019, 359, 274–280. [Google Scholar] [CrossRef]
- Hamilton, T.J.; Morrill, A.; Lucas, K.; Gallup, J.; Harris, M.; Healey, M.; Pitman, T.; Schalomon, M.; Digweed, S.; Tresguerres, M. Establishing zebrafish as a model to study the anxiolytic effects of scopolamine. Sci. Rep. 2017, 7, 15081. [Google Scholar] [CrossRef] [Green Version]
- Richetti, S.K.; Blank, M.; Capiotti, K.M.; Piato, A.L.; Bogo, M.R.; Vianna, M.R.; Bonan, C.D. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav. Brain Res. 2012, 16, 198–206. [Google Scholar] [CrossRef]
- Cho, H.; Lee, C.J.; Choi, J.; Hwang, J.; Lee, Y. Anxiolytic effects of an acetylcholinesterase inhibitor, physostigmine, in the adult zebrafish. Animal Cells Syst. 2020, 9, 1083. [Google Scholar] [CrossRef]
- Levin, E.D.; Bencan, Z.; Cerutti, D.T. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 2007, 90, 54–58. [Google Scholar] [CrossRef]
- Bencan, Z.; Levin, E.D. The role of α7 and α4β2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. Physiol. Behav. 2008, 95, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Zeitlin, R.; Patel, S.; Solomon, R.; Tran, J.; Weeber, E.J.; Echeverria, V. Cotinine enhances the extinction of contextual fear memory and reduces anxiety after fear conditioning. Behav. Brain Res. 2012, 228, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Ioniță, R.; Valu, V.M.; Postu, P.A.; Cioancă, O.; Hrițcu, L.; Mihasan, M. 6-hydroxy-l-nicotine effects on anxiety and depression in a rat model of chlorisondamine. Farmacia 2017, 65, 237–240. [Google Scholar]
- Khan, K.M.; Collier, A.D.; Meshalkina, D.A.; Kysil, E.V.; Khatsko, S.L.; Kolesnikova, T.; Morzherin, Y.Y.; Warnick, J.E.; Kalueff, A.V.; Echevarria, D.J. Zebrafish models in neuropsychopharmacology and CNS drug discovery. Br. J. Pharmacol. 2017, 174, 1925–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, A.K.; Dandapat, J.; Dash, U.C.; Kanhar, S. Features and outcomes of drugs for combination therapy as multi-targets strategy to combat Alzheimer’s disease. J. Ethnopharmacol. 2018, 215, 42–73. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zheng, X.; Fang, T.; Yang, X.; Luo, X.; Guo, A.; Newell, K.A.; Huang, X.F.; Yu, Y. Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J. Neuroinflamm. 2018, 15, 112. [Google Scholar] [CrossRef] [Green Version]
- May, Z.; Morrill, A.; Holcombe, A.; Johnston, T.; Gallup, J.; Fouad, K.; Schalomon, M.; Hamilton, T.J. Object recognition memory in zebrafish. Behav. Brain Res. 2016, 296, 199–210. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Dadda, M. Assessing memory in zebrafish using the one-trial test. Behav. Processes 2014, 106, 1–4. [Google Scholar] [CrossRef]
- Weinberger, N.M. Food for thought: Honeybee foraging, memory, and acetylcholine. Sci. STKE 2006, 2006, pe23. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Levin, E.D.; Chen, E. Nicotinic involvement in memory function in zebrafish. Neurotoxicol. Teratol. 2004, 26, 731–735. [Google Scholar] [CrossRef]
- Levin, E.D.; Limpuangthip, J.; Rachakonda, T.; Peterson, M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology 2006, 184, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Eddins, D.; Petro, A.; Williams, P.; Cerutti, D.T.; Levin, E.D. Nicotine effects on learning in zebrafish: The role of dopaminergic systems. Psychopharmacology 2009, 202, 103–109. [Google Scholar] [CrossRef] [PubMed]
- de Castro, M.R.; Lima, J.V.; Salomão de Freitas, D.P.; de Souza Valente, R.; Dummer, N.S.; de Aguiar, R.B.; dos Santos, L.C.; Marins, L.F.; Geracitano, L.A.; Monserrat, J.M.; et al. Behavioral and neurotoxic effects of arsenic exposure in zebrafish (Danio rerio, Teleostei: Cyprinidae). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Braida, D.; Ponzoni, L.; Martucci, R.; Sparatore, F.; Gotti, C.; Sala, M. Role of neuronal nicotinic acetylcholine receptors (nAChRs) on learning and memory in zebrafish. Psychopharmacology 2014, 231, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Stefan, M.; Brandsch, R.; Mihasan, M.; Hrițcu, L.; Ștefan, M.; Brandsch, R.; Mihășan, M. 6-hydroxy-l-nicotine from Arthrobacter nicotinovorans sustain spatial memory formation by decreasing brain oxidative stress in rats. J. Physiol. Biochem. 2013, 69, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Dubey, R. Target Enzyme in Alzheimer’s Disease: Acetylcholinesterase Inhibitors. Curr. Top. Med. Chem. 2019, 19, 264–275. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Capatina, L.; Todirascu-Ciornea, E.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Dumitru, G. Thymus vulgaris Essential Oil Protects Zebrafish against Cognitive Dysfunction by Regulating Cholinergic and Antioxidants Systems. Antioxidants 2020, 9, 1083. [Google Scholar] [CrossRef]
- Todirascu-Ciornea, E.; El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Boiangiu, R.S.; Dumitru, G.; Hritcu, L.; Singab, A.N.B. Schinus terebinthifolius Essential Oil Attenuates Scopolamine-Induced Memory Deficits via Cholinergic Modulation and Antioxidant Properties in a Zebrafish Model. Evid. Based Complement. Altern. Med. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative Stress, Amyloid-β Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimers. Dis. 2018, 62, 1345–1367. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-T.; Chang, W.-N.; Tsai, N.-W.; Huang, C.-C.; Kung, C.-T.; Su, Y.-J.; Lin, W.-C.; Cheng, B.-C.; Su, C.-M.; Chiang, Y.-F.; et al. The roles of biomarkers of oxidative stress and antioxidant in Alzheimer’s disease: A systematic review. Biomed Res. Int. 2014, 2014, 182303. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadegan, M.; Soodi, M. Comparison of memory impairment and oxidative stress following single or repeated doses administration of scopolamine in rat hippocampus. Basic Clin. Neurosci. 2018, 9, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionita, R.; Postu, P.A.; Beppe, G.J.; Mihasan, M.; Petre, B.A.; Hancianu, M.; Cioanca, O.; Hritcu, L. Cognitive-enhancing and antioxidant activities of the aqueous extract from Markhamia tomentosa (Benth.) K. Schum. stem bark in a rat model of scopolamine. Behav. Brain Funct. 2017, 13, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S.I.; Kim, M.O. Melatonin Rescue Oxidative Stress-Mediated Neuroinflammation/Neurodegeneration and Memory Impairment in Scopolamine-Induced Amnesia Mice Model. J. Neuroimmune Pharmacol. 2019, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Lv, J.; Dong, L.; Jiang, N.; Wang, Y.; Wang, Q.; Li, Y.; Chen, S.; Fan, B.; Wang, F.; et al. Neuroprotective effects of 20(S)-protopanaxatriol (PPT) on scopolamine-induced cognitive deficits in mice. Phyther. Res. 2018, 32, 1056–1063. [Google Scholar] [CrossRef]
- Nathiga Nambi, K.S.; Abdul Majeed, S.; Taju, G.; Sivasubbu, S.; Sarath Babu, V.; Sahul Hameed, A.S. Effects of nicotine on zebrafish: A comparative response between a newly established gill cell line and whole gills. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 195, 68–77. [Google Scholar] [CrossRef]
- Srivastava, E.D.; Hallett, M.B.; Rhodes, J. Effect of Nicotine and Cotinine on the Production of Oxygen Free Radicals by Neutrophils in Smokers and Non-smokers. Hum. Toxicol. 1989, 8, 461–463. [Google Scholar] [CrossRef]
- Soto-Otero, R.; Méndez-Alvarez, E.; Hermida-Ameijeiras, A.; López-Real, A.M.; Labandeira-García, J.L. Effects of (-)-nicotine and (-)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: Relevance for Parkinson’s disease. Biochem. Pharmacol. 2002, 64, 125–135. [Google Scholar] [CrossRef]
- de Aguiar, R.B.; Parfitt, G.M.; Jaboinski, J.; Barros, D.M. Neuroactive effects of cotinine on the hippocampus: Behavioral and biochemical parameters. Neuropharmacology 2013, 71, 292–298. [Google Scholar] [CrossRef]
- Mihășan, M.; Căpățînă, L.; Neagu, E.; Ștefan, M.; Hrițcu, L. In-silico identification of 6-hydroxy-L-nicotine as a novel neuroprotective drug. Rom. Biotechnol. Lett. 2013, 18, 8333–8340. [Google Scholar]
- Mocanu, E.M.; Mazarachi, A.L.; Mihasan, M. In vitro stability and antioxidant potential of the neuprotective metabolite 6-hydroxy-nicotine. J. Exp. Mol. Biol. 2018, 19, 53–58. [Google Scholar]
- Tanila, H. The role of BDNF in Alzheimer’s disease. Neurobiol. Dis. 2017, 97, 114–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.H.; Park, J.H.; Lee, T.K.; Song, M.; Kim, H.; Lee, J.C.; Kim, Y.M.; Lee, C.H.; Hwang, I.K.; Kang, I.J.; et al. Melatonin attenuates scopolamine-induced cognitive impairment via protecting against demyelination through BDNF-TrkB signaling in the mouse dentate gyrus. Chem. Biol. Interact. 2018, 285, 8–13. [Google Scholar] [CrossRef]
- Machaalani, R.; Chen, H. Brain derived neurotrophic factor (BDNF), its tyrosine kinase receptor B (TrkB) and nicotine. Neurotoxicology 2018, 65, 186–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majdi, A.; Sadigh-Eteghad, S.; Talebi, M.; Farajdokht, F.; Erfani, M.; Mahmoudi, J.; Gjedde, A. Nicotine Modulates Cognitive Function in D-Galactose-Induced Senescence in Mice. Front. Aging Neurosci. 2018, 10, 1–13. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Vatandoust, S.M.; Mahmoudi, J.; Rahigh Aghsan, S.; Majdi, A. Cotinine ameliorates memory and learning impairment in senescent mice. Brain Res. Bull. 2020, 164, 65–74. [Google Scholar] [CrossRef]
- Gao, J.; Adam, B.L.; Terry, A.V. Evaluation of nicotine and cotinine analogs as potential neuroprotective agents for Alzheimer’s disease. Bioorganic Med. Chem. Lett. 2014, 24, 1472–1478. [Google Scholar] [CrossRef] [Green Version]
- Burgess, S.; Zeitlin, R.; Echeverria, V. Cotinine Inhibits Amyloid-β Peptide Neurotoxicity and Oligomerization. J. Clin. Toxicol. 2012, 1, 2011–2013. [Google Scholar] [CrossRef]
- Riveles, K.; Huang, L.Z.; Quik, M. Cigarette smoke, nicotine and cotinine protect against 6-hydroxydopamine-induced toxicity in SH-SY5Y cells. Neurotoxicology 2008, 29, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Reichmann, F.; Holzer, P. Neuropeptide Y: A stressful review. Neuropeptides 2016, 55, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Koide, S.; Onishi, H.; Hashimoto, H.; Kai, T.; Yamagami, S. Plasma neuropeptide Y is reduced in patients with Alzheimer’s disease. Neurosci. Lett. 1995, 198, 149–151. [Google Scholar] [CrossRef]
- Albuquerque, M.S.; Mahar, I.; Davoli, M.A.; Chabot, J.-G.; Mechawar, N.; Quirion, R.; Krantic, S. Regional and sub-regional differences in hippocampal GABAergic neuronal vulnerability in the TgCRND8 mouse model of Alzheimer’s disease. Front. Aging Neurosci. 2015, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flood, J.F.; Hernandez, E.N.; Morley, J.E. Modulation of memory processing by neuropeptide Y. Brain Res. 1987, 421, 280–290. [Google Scholar] [CrossRef]
- Li, M.D.; Kane, J.K.; Parker, S.L.; McAllen, K.; Matta, S.G.; Sharp, B.M. Nicotine administration enhances NPY expression in the rat hypothalamus. Brain Res. 2000, 867, 157–164. [Google Scholar] [CrossRef]
- Rangani, R.J.; Upadhya, M.A.; Nakhate, K.T.; Kokare, D.M.; Subhedar, N.K. Nicotine evoked improvement in learning and memory is mediated through NPY Y1 receptors in rat model of Alzheimer’s disease. Peptides 2012, 33, 317–328. [Google Scholar] [CrossRef]
- McFadden, K.L.; Cornier, M.-A.; Tregellas, J.R. The role of alpha-7 nicotinic receptors in food intake behaviors. Front. Psychol. 2014, 5, 553. [Google Scholar] [CrossRef] [Green Version]
- Lesuis, S.L.; Hoeijmakers, L.; Korosi, A.; de Rooij, S.R.; Swaab, D.F.; Kessels, H.W.; Lucassen, P.J.; Krugers, H.J. Vulnerability and resilience to Alzheimer’s disease: Early life conditions modulate neuropathology and determine cognitive reserve. Alzheimers. Res. Ther. 2018, 10, 95. [Google Scholar] [CrossRef]
- Zhu, Q.B.; Unmehopa, U.; Bossers, K.; Hu, Y.T.; Verwer, R.; Balesar, R.; Zhao, J.; Bao, A.M.; Swaab, D. MicroRNA-132 and early growth response-1 in nucleus basalis of Meynert during the course of Alzheimer’s disease. Brain 2016, 139, 908–921. [Google Scholar] [CrossRef]
- Srivas, S.; Thakur, M.K. Transcriptional co-repressor SIN3A silencing rescues decline in memory consolidation during scopolamine-induced amnesia. J. Neurochem. 2018, 145, 204–216. [Google Scholar] [CrossRef] [Green Version]
- Dunckley, T.; Lukas, R.J. Nicotine modulates the expression of a diverse set of genes in the neuronal SH-SY5Y cell line. J. Biol. Chem. 2003, 278, 15633–15640. [Google Scholar] [CrossRef] [Green Version]
- Belluardo, N.; Olsson, P.A.; Mudo’, G.; Sommer, W.H.; Amato, G.; Fuxe, K. Transcription factor gene expression profiling after acute intermittent nicotine treatment in the rat cerebral cortex. Neuroscience 2005, 133, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zhu, L.; Zhang, J.; Qiu, J.; Du, G.; Qiao, Z.; Jin, G.; Gao, F.; Zhang, Q. Low Dose Nicotine Attenuates Aβ Neurotoxicity through Activation Early Growth Response Gene 1 Pathway. PLoS ONE 2015, 10, e0120267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, H.; Tanji, K.; Wakabayashi, K.; Matsuura, S.; Itoh, K. Role of the Keap1/Nrf2 pathway in neurodegenerative diseases. Pathol. Int. 2015, 65, 210–219. [Google Scholar] [CrossRef]
- Ramsey, C.P.; Glass, C.A.; Montgomery, M.B.; Lindl, K.A.; Ritson, G.P.; Chia, L.A.; Hamilton, R.L.; Chu, C.T.; Jordan-Sciutto, K.L. Expression of Nrf2 in neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2007, 66, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Wang, Z.; Luo, Y.; Zhang, Y.; He, W.; Mei, Y.; Xue, J.; Li, M.; Pan, H.; Li, W.; et al. FA-97, a New Synthetic Caffeic Acid Phenethyl Ester Derivative, Protects against Oxidative Stress-Mediated Neuronal Cell Apoptosis and Scopolamine-Induced Cognitive Impairment by Activating Nrf2/HO-1 Signaling. Oxid. Med. Cell. Longev. 2019, 2019, 1–21. [Google Scholar] [CrossRef]
- Venkatesan, R.; Subedi, L.; Yeo, E.J.; Kim, S.Y. Lactucopicrin ameliorates oxidative stress mediated by scopolamine-induced neurotoxicity through activation of the NRF2 pathway. Neurochem. Int. 2016, 99, 133–146. [Google Scholar] [CrossRef]
- Kasnak, G.; Könönen, E.; Syrjänen, S.; Gürsoy, M.; Zeidán-Chuliá, F.; Firatli, E.; Gürsoy, U.K. NFE2L2/NRF2, OGG1, and cytokine responses of human gingival keratinocytes against oxidative insults of various origin. Mol. Cell. Biochem. 2019, 452, 63–70. [Google Scholar] [CrossRef]
- Lee, H.-J.; Pi, S.-H.; Kim, Y.; Kim, H.-S.; Kim, S.-J.; Kim, Y.-S.; Lee, S.-K.; Kim, E.-C. Effects of Nicotine on Antioxidant Defense Enzymes and RANKL Expression in Human Periodontal Ligament Cells. J. Periodontol. 2009, 80, 1281–1288. [Google Scholar] [CrossRef]
- Pardo, M.; Beurel, E.; Jope, R.S. Cotinine administration improves impaired cognition in the mouse model of Fragile X syndrome. Eur. J. Neurosci. 2017, 45, 490–498. [Google Scholar] [CrossRef] [Green Version]








| Gene | Product Size (bp) | Primer | Sequence | Reference Sequence |
|---|---|---|---|---|
| npy | 104 | Forward | 5′-GAC TCT CAC AGA AGG GTA TCC-3′ | NM_131074.2 |
| Reverse | 5′-GGT TGA TGT AGT GTC TTA GTG CTG-3′ | |||
| bdnf | 83 | Forward | 5′-GCT CTC TCA ATG CGC ACT AC-3′ | NM_131595.2 |
| Reverse | 5′-TGA CTG AGC GGA TCC TTT GG-3′ | |||
| egr1 | 110 | Forward | 5′-AGT TTG ATC ACC TTG CTG GAG-3′ | NM_131248.1 |
| Reverse | 5′-AAC GGC CTG TGT AAG ATA TGG-3′ | |||
| nrf2a | 106 | Forward | 5′-ATG TCT AAA ATG CAG CCA AGC C-3′ | NM_182889.1 |
| Reverse | 5′-CGG TAG CTG AAG TCG AAC AC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boiangiu, R.S.; Mihasan, M.; Gorgan, D.L.; Stache, B.A.; Hritcu, L. Anxiolytic, Promnesic, Anti-Acetylcholinesterase and Antioxidant Effects of Cotinine and 6-Hydroxy-L-Nicotine in Scopolamine-Induced Zebrafish (Danio rerio) Model of Alzheimer’s Disease. Antioxidants 2021, 10, 212. https://doi.org/10.3390/antiox10020212
Boiangiu RS, Mihasan M, Gorgan DL, Stache BA, Hritcu L. Anxiolytic, Promnesic, Anti-Acetylcholinesterase and Antioxidant Effects of Cotinine and 6-Hydroxy-L-Nicotine in Scopolamine-Induced Zebrafish (Danio rerio) Model of Alzheimer’s Disease. Antioxidants. 2021; 10(2):212. https://doi.org/10.3390/antiox10020212
Chicago/Turabian StyleBoiangiu, Razvan Stefan, Marius Mihasan, Dragos Lucian Gorgan, Bogdan Alexandru Stache, and Lucian Hritcu. 2021. "Anxiolytic, Promnesic, Anti-Acetylcholinesterase and Antioxidant Effects of Cotinine and 6-Hydroxy-L-Nicotine in Scopolamine-Induced Zebrafish (Danio rerio) Model of Alzheimer’s Disease" Antioxidants 10, no. 2: 212. https://doi.org/10.3390/antiox10020212