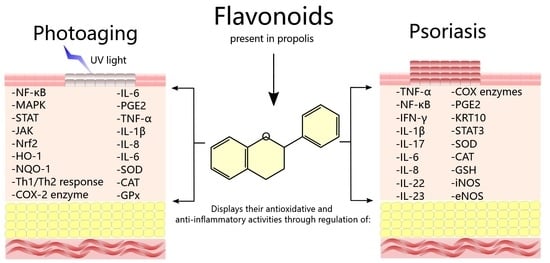
Flavonoids Present in Propolis in the Battle against Photoaging and Psoriasis
Abstract
:1. Introduction
2. Anti-Photoaging and Photoprotective Properties of Flavonoids
2.1. Flavones
| Flavones | Model/UV Radiation | Activities | Ref. |
|---|---|---|---|
| Luteolin, chrysin, and apigenin | HDFs/UVA | All flavonoids inhibit the MMP-1 mRNA levels; presents antioxidant activity | [50] |
| Luteolin | Decreases the MMP-1 expression, the IL-6 secretion, and the hyaluronidase activity | [51] | |
| HaCaT cells/SSR | Suppresses the IL-20 production; the HaCaT supernatants pretreated and added to fibroblasts decrease MMP-1 and IL-6 expression | ||
| Skin explants/SSR | Decreases MMP-1 and IL-6 expression | ||
| HaCaT cells/UVB | Inhibits CPDs and ROS production; suppresses the p38 MAPK and ERK activation; decreases COX-2 expression and the PGE2 synthesis | [53] | |
| Suppresses MMP-1 expression, AP-1 and c-Fos activation, c-Jun phosphorylation, p90RSK and JNK1 activity, and MMP-1 expression | [54] | ||
| Luteolin and apigenin | HaCaT cells/UVA | Both suppress ROS generation and MMP-1 production; inhibit c-Jun and c-Fos expression and the MAP kinases phosphorylation; reduce the influx of Ca2+ and Ca2+/calmodulin-dependent kinases phosphorylation | [55] |
| Chrysin | HDFs/UVB | Increases collagen I secretion and the GSH level; decreases the MDA and MMP-1 level | [38] |
| HaCaT cells/UVA and UVB | Decreases ROS generation, COX-2 expression, and apoptosis; increases AQP-3 expression; suppresses p38 and JNK activation | [58] | |
| Baicalein | HDFs/UVB | Inhibits the 12-lipoxygenase and through the TRPV1-Ca-ERK pathway suppresses the MMP-1 expression | [59] |
| Baicalin | Reduces the p16INK−4a, p21WAF−1, p53, and γ-H2AX proteins level | [60] | |
| C57BL/6 mice/UVB | Decreases skin thickening; reduces MMP-3 and MMP-1 expression; increases collagen III and I production | ||
| HDFs/UVA | Increases the telomere length, the TGF-β1 secretion, the GPx and SOD levels, and the c-myc mRNA expression and protein level; decreases MDA levels, p16, p53, p66, TIMP-1, MMP-1 mRNA expression, and the p16 and p53 proteins level | [61] | |
| HaCaT cells/UVB | Suppresses the CPDs and apoptosis; reduces the c-fos and p53/p21 mRNA expression, the p53, the RPA, and PCNA proteins level, and TNF-α and IL-6 secretion | [62] | |
| BALB/c mice/UVB | Reduces skin hyperplasia and edema, hydrogen peroxide generation, and photolesions formation | [64] | |
| C3H/HeN mice/UVA | Decreases the CD11b+Gr1+ myeloid-derived suppressor cells, the TLR4 expression level, TRAF6, IRAK4, and MyD88 protein expression, TIRAP and MyD88 mRNA expression, MMP-1 and MMP-9 expression levels, COX-2, IL-1β, IL-10, and iNOS | [65] |
2.2. Flavonols
2.3. Flavanones
| Flavanones | Model/UV Radiation | Activities | Ref. |
|---|---|---|---|
| Hesperetin | HDFs/UVA | Decreases ROS generation; reduces collagen depletion and MMP-1 activity; positively regulates Nrf2 activity and its genes NQO-1 and GST | [82] |
| BALB/c mice/UVA | Decreases collagen loss, MMP-1 activity, and 8-OhdG; increases nuclear Nrf2 levels and its NQO-1 and GST target proteins | ||
| Hesperidin | HaCaT cells/UVA | Increases SOD activity and total antioxidative capacity levels; reduces MDA content; decreases IL-1β, IL-6, and TNF-α mRNA levels and proteins expression | [83] |
| Hairless mice/UVB | Decreases collagen fiber loss, wrinkle formation, and TEWL; suppresses MMP-9 activity and mRNA levels, TNF-α and IL-8 production, and ERK and MEK phosphorylation | [84] | |
| Naringenin | HaCaT cells/UVB | Suppresses MMP-1 expression, AP-1 activity, FRA1 expression and phosphorylation, p90RSK phosphorylation, and ERK2 activity | [85] |
| Hairless mice/UVB | Suppresses TEWL, wrinkle formation, and MMP-13 expression | ||
| Inhibits the IL-1β, IL-10, IL-6, and TNF-α production; suppresses lipid hydroperoxides and superoxide anion production; preserves GSH levels and CAT activity, Nrf2 mRNA expression, glutathione reductase, and GPx | [86] |
2.4. Flavan-3-ols and Isoflavones
| Flavonoids | Model/UV Radiation | Activities | Ref. |
|---|---|---|---|
| EGCG and EGCG nanoparticles | HaCaT cells/UVB | Both decrease the intracellular ROS levels, MDA level, and MMP-2 and MMP-9 expression | [88] |
| EGCG | Reduces apoptosis; suppresses c-fos, p21, and p53 mRNA expression; decreases TNF-α and IL-6 secretion | [89] | |
| HDFs/UVB | Inhibits collagen degradation, MAPK, ERK1/2, p38 MAPK, and JNK phosphorylation and ASK-1 activation; the pre-administration suppresses MMP-13, MMP-8, and MMP-1 production | [90] | |
| Rats/UVA | Diminution in sunburn cells and dermo-epidermal activation | [91] | |
| Catechin | Increases CAT, GPx, and SOD levels; decreases TBRAS levels | [96] | |
| Genistein | HDFs/UVB | Reduces the apoptosis and MDA level; increases SOD activity; decreases the p66Shc and FKHRL1 level and phosphorylation | [97] |
2.5. Propolis: Bee Product Rich in Flavonoids with Anti-Photoaging Activity
3. Properties of Flavonoids against Psoriasis
3.1. Flavones
| Flavones | Model/Psoriasis Inducer | Activities | Ref. |
|---|---|---|---|
| Luteolin | NHEKs and HaCaT cells | Inhibits mRNA expression of IL-8 and IL-6; reduces mRNA expression of NFKB1 and RELA and HaCaT cell proliferation | [120] |
| HaCaT cells | Inhibits transcriptional expression of HSP90β and HSP90α; decreases exosomes amount | [121] | |
| BALB/c mice/ imiquimod | Reduces psoriatic area, PASI, histological damage, and HSP90β and HSP90α expression; decreases the proportion of Th17/Treg cells and Th1/Th2 | ||
| Raw264.7 macrophages | Decreases both mRNA and protein levels of TNF-α, IL-1β, IL-6, IL-23, and IL-17A; inhibits NF-κB p65 and COX-2 expression; suppresses iNOS and NO | [39] | |
| BALB/c mice/ imiquimod | Reduces PASI, infiltration of macrophage, T cells, and neutrophil, and IL-1β, IL-17A, IL-23, and IL-6 expression | ||
| Luteolin-7-glucoside | HEKn | Modifies cell cycle, energy, fatty acid, and redox metabolism; increases KRT10 expression and cortisol levels; decreases PGE2 level | [122] |
| C57BL/6 mice/imiquimod | Reduces skin psoriasis-like lesions, Ki67 and p63; increases KRT10, TGase-1, and Loricrin; modulates expression of STAT3 | ||
| Baicalein | HaCaT cells | Inhibits cell growth; causes growth arrest in G0/G1phase; induces morphological differentiation Ca2+ dependent; increases KRT10 and KRT1 expression and ERK phosphorylation; activates TRPV4 | [123] |
| Baicalin | BALB/c and ICR mice/DNFB | Reduces psoriatic symptoms, edema, and inflammatory cell infiltration; increases orthokeratosis | [124] |
| BALB/c mice/imiquimod | Reduces psoriatic symptoms, PASI, histological damage, expression of TNF-α, IL-23, IL-22, and IL-17A, and γδT cells infiltration | [116] | |
| Chrysin | NHEKs | Attenuates phosphorylation of JNK, ERK, and p38 kinase; decreases p-STAT3 and p-JAK2; suppresses CCL20 and AMPs expression | [35] |
| BALB/c mice/imiquimod | Reduces psoriatic symptoms, PASI, histological damage, TEWL, erythema, blood flow, and thickness; increases the content of surface skin hydration | ||
| Tangeretin | HaCaT cells | Inhibits the nuclear translocation of NF-κB p65 and HIF-1α | [125] |
| BALB/c mice/PMA-induced ear inflammation | Reduces psoriatic symptoms, histological damage, PMA-induced hyperplasia; down-regulates the production of IL-1β, IL-6, IL-4, TNF-α, IFN-γ, PGE2, COX-2, MIP-2, MCP-1, KC, TRX, nNOS, iNOS, eNOS, MMP-9, MMP-2, TLR4, VEGF, p-Akt, p-p38, p-JNK, p-ERK1/2, NF-κB p65, NF-κB p50, IκBα, IKK-γ, and HIF-1α; suppresses MDA, and NO expression; increases SOD-2, HO-1, and CAT expression |
3.2. Flavonols
3.3. Flavanones, Flavan-3-ols, and Isoflavones
| Flavonoids | Model/Psoriasis Inducer | Activities | Ref. |
|---|---|---|---|
| Naringenin | hPBMCs | Suppress LPS-induced serum TNF-α levels | [128] |
| BALB/c mice/imiquimod | Reduces psoriatic symptoms, PASI, histological damage, and TEWL; inhibits neutrophil migration and IL-6 | [8] | |
| Hesperidin | HaCaT cells | Inhibits LPS-induced cell proliferation; down-regulates expression of p-ERK1/2 | [129] |
| BALB/c mice/imiquimod | Reduces psoriatic symptoms, PASI, histological damage, expression of involucrin, IL-22, IL-23, IL-17, TNF-α, and IL-1β; decreases p-ERK1/2 level | ||
| EGCG and EGCG nanoparticles | NHEKs | Induces differentiation; increases involucrin, TGase-1, KRT10, and caspase-14; inhibits CXCL2, TGF-β, TNF-α, IL-8, IL-6, and IL-1β | [130] |
| BALB/c mice/imiquimod | Reduces psoriatic symptoms and loricrin expression; decreases Ki67 expression, infiltration CD4+ T lymphocytes, and tissue vascularization; restores JunB and KRT10 expression; inhibits expression of IL-1β and TNF-α | ||
| EGCG | Reduces psoriatic symptoms, PASI, histological damage, lipoperoxidation; decreases CD4+ T cells infiltration, IL- 23, IL-22, IL-17F, and IL-17A levels; increases CAT and SOD bioactivities | [131] | |
| Genistein | HaCaT cells | Decreases MCP-1, VEGFA, TNF-α, IL-23, IL-8, and IL-1β; inhibits IκBα phosphorylation; decreases NF-κB level | [132] |
| BALB/c mice/imiquimod | Reduces histological damage, CD45 inflammatory cell infiltration, TNF-α, IL-6, IL-1β, CCL2, IL-23, and IL-17; suppresses STAT3 phosphorylation |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baek, J.; Lee, M.-G. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016, 21, 164–169. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M. The roles of vitamin c in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [Green Version]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Afaq, F.; Mukhtar, H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp. Dermatol. 2006, 15, 678–684. [Google Scholar] [CrossRef]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, uva induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Nayak, A.; Narayan, R.; Nayak, U.Y. Anti-aging and sunscreens: Paradigm shift in cosmetics. Adv. Pharm. Bull. 2019, 9, 348. [Google Scholar] [CrossRef] [Green Version]
- Alalaiwe, A.; Lin, C.-F.; Hsiao, C.-Y.; Chen, E.-L.; Lin, C.-Y.; Lien, W.-C.; Fang, J.-Y. Development of flavanone and its derivatives as topical agents against psoriasis: The prediction of therapeutic efficiency through skin permeation evaluation and cell-based assay. Int. J. Pharm. 2020, 581, 119256. [Google Scholar] [CrossRef]
- El-Gammal, A.; Di Nardo, V.; Daaboul, F.; Tchernev, G.; Wollina, U.; Lotti, J.; Lotti, T. Is there a place for local natural treatment of psoriasis? Open Access Maced. J. Med Sci. 2018, 6, 839. [Google Scholar] [CrossRef] [Green Version]
- Kocic, H.; Damiani, G.; Stamenkovic, B.; Tirant, M.; Jovic, A.; Tiodorovic, D.; Peris, K. Dietary compounds as potential modulators of microrna expression in psoriasis. Ther. Adv. Chronic Dis. 2019, 10, 2040622319864805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barygina, V.; Becatti, M.; Lotti, T.; Taddei, N.; Fiorillo, C. Low dose cytokines reduce oxidative stress in primary lesional fibroblasts obtained from psoriatic patients. J. Dermatol. Sci. 2016, 83, 242–244. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [Green Version]
- Kerdel, F.; Zaiac, M. An evolution in switching therapy for psoriasis patients who fail to meet treatment goals. Dermatol. Ther. 2015, 28, 390–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid. -Based Complementary Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Fokt, H.; Pereira, A.; Ferreira, A.; Cunha, A.; Aguiar, C. How do bees prevent hive infections? The antimicrobial properties of propolis. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 1, 481–493. [Google Scholar]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.A.E.; Amarante, M.K.; Conti, B.J.; Sforcin, J.M. Cytotoxic constituents of propolis inducing anticancer effects: A review. J. Pharm. Pharmacol. 2011, 63, 1378–1386. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant activity in bee products: A review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Osés, S.M.; Marcos, P.; Azofra, P.; de Pablo, A.; Fernández-Muíño, M.Á.; Sancho, M.T. Phenolic profile, antioxidant capacities and enzymatic inhibitory activities of propolis from different geographical areas: Needs for analytical harmonization. Antioxidants 2020, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Zaccaria, V.; Curti, V.; Di Lorenzo, A.; Baldi, A.; Maccario, C.; Sommatis, S.; Mocchi, R.; Daglia, M. Effect of green and brown propolis extracts on the expression levels of micrornas, mrnas and proteins, related to oxidative stress and inflammation. Nutrients 2017, 9, 1090. [Google Scholar] [CrossRef]
- Tiveron, A.P.; Rosalen, P.L.; Franchin, M.; Lacerda, R.C.C.; Bueno-Silva, B.; Benso, B.; Denny, C.; Ikegaki, M.; Alencar, S.M.D. Chemical characterization and antioxidant, antimicrobial, and anti-inflammatory activities of south brazilian organic propolis. PLoS ONE 2016, 11, e0165588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Yañez, N.; Rivera-Yañez, C.R.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Reyes-Reali, J.; Mendoza-Ramos, M.I.; Méndez-Cruz, A.R.; Nieto-Yañez, O. Effects of propolis on infectious diseases of medical relevance. Biology 2021, 10, 428. [Google Scholar] [CrossRef]
- Kitamura, H. Effects of propolis extract and propolis-derived compounds on obesity and diabetes: Knowledge from cellular and animal models. Molecules 2019, 24, 4394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wezgowiec, J.; Wieczynska, A.; Wieckiewicz, W.; Kulbacka, J.; Saczko, J.; Pachura, N.; Wieckiewicz, M.; Gancarz, R.; Wilk, K.A. Polish propolis—Chemical composition and biological effects in tongue cancer cells and macrophages. Molecules 2020, 25, 2426. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Yañez, N.; Rivera-Yañez, C.R.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Méndez-Cruz, A.R.; Nieto-Yañez, O. Biomedical properties of propolis on diverse chronic diseases and its potential applications and health benefits. Nutrients 2021, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Šuran, J.; Cepanec, I.; Mašek, T.; Radić, B.; Radić, S.; Tlak Gajger, I.; Vlainić, J. Propolis extract and its bioactive compounds—from traditional to modern extraction technologies. Molecules 2021, 26, 2930. [Google Scholar] [CrossRef] [PubMed]
- Šuran, J.; Cepanec, I.; Mašek, T.; Starčević, K.; Tlak Gajger, I.; Vranješ, M.; Radić, B.; Radić, S.; Kosalec, I.; Vlainić, J. Nonaqueous polyethylene glycol as a safer alternative to ethanolic propolis extracts with comparable antioxidant and antimicrobial activity. Antioxidants 2021, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Gajger, I.T.; PAVLOVIĆ, I.; BOJIĆ, M.; Kosalec, I.; SREČEC, S.; VLAINIĆ, T.; VLAINIĆ, J. The components responsible for the antimicrobial activity of propolis from continental and mediterranean regions in croatia. Czech J. Food Sci. 2017, 35, 376–385. [Google Scholar]
- Marzocchella, L.; Fantini, M.; Benvenuto, M.; Masuelli, L.; Tresoldi, I.; Modesti, A.; Bei, R. Dietary flavonoids: Molecular mechanisms of action as anti-inflammatory agents. Recent Pat. Inflamm. Allergy Drug Discov. 2011, 5, 200–220. [Google Scholar] [CrossRef]
- Li, H.-J.; Wu, N.-L.; Pu, C.-M.; Hsiao, C.-Y.; Chang, D.-C.; Hung, C.-F. Chrysin alleviates imiquimod-induced psoriasis-like skin inflammation and reduces the release of ccl20 and antimicrobial peptides. Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Arct, J.; Pytkowska, K. Flavonoids as components of biologically active cosmeceuticals. Clin. Dermatol. 2008, 26, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.-G.; Byun, S. Quercetin directly targets jak2 and pkcδ and prevents uv-induced photoaging in human skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Lu, Y.; Yu, W.-G.; Zhao, X.; Lu, Y.-H. Anti-photoageing and anti-melanogenesis activities of chrysin. Pharm. Biol. 2016, 54, 2692–2700. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Hu, M.; Zang, X.; Liu, Q.; Du, J.; Hu, J.; Zhang, L.; Du, Z.; Xiang, Z. Luteolin attenuates imiquimod–induced psoriasis-like skin lesions in balb/c mice via suppression of inflammation response. Biomed. Pharmacother. 2020, 131, 110696. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.; DiPietro, L.A. Aging and wound healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Schuger, L.; Dame, M.K.; Leonard, C.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photodamaged skin. J. Investig. Dermatol. 2004, 122, 1471–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [Green Version]
- Nakyai, W.; Saraphanchotiwitthaya, A.; Viennet, C.; Humbert, P.; Viyoch, J. An in vitro model for fibroblast photoaging comparing single and repeated uva irradiations. Photochem. Photobiol. 2017, 93, 1462–1471. [Google Scholar] [CrossRef]
- Besaratinia, A.; Kim, S.-I.; Pfeifer, G.P. Rapid repair of uva-induced oxidized purines and persistence of uvb-induced dipyrimidine lesions determine the mutagenicity of sunlight in mouse cells. FASEB J. 2008, 22, 2379–2392. [Google Scholar] [CrossRef] [Green Version]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free. Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Sim, G.-S.; Lee, B.-C.; Cho, H.S.; Lee, J.W.; Kim, J.-H.; Lee, D.-H.; Kim, J.-H.; Pyo, H.-B.; Moon, D.C.; Oh, K.-W. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in uva-irradiated human dermal fibroblast. Arch. Pharmacal Res. 2007, 30, 290–298. [Google Scholar] [CrossRef]
- Wölfle, U.; Heinemann, A.; Esser, P.R.; Haarhaus, B.; Martin, S.F.; Schempp, C.M. Luteolin prevents solar radiation-induced matrix metalloproteinase-1 activation in human fibroblasts: A role for p38 mitogen-activated protein kinase and interleukin-20 released from keratinocytes. Rejuvenation Res. 2012, 15, 466–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, D.; Baxter, B.; Campbell, B.C.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Wölfle, U.; Esser, P.R.; Simon-Haarhaus, B.; Martin, S.F.; Lademann, J.; Schempp, C.M. Uvb-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free. Radic. Biol. Med. 2011, 50, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Jung, S.K.; Byun, S.; Lee, E.J.; Hwang, J.A.; Seo, S.G.; Kim, Y.A.; Yu, J.G.; Lee, K.W.; Lee, H.J. Luteolin suppresses uvb-induced photoageing by targeting jnk1 and p90rsk2. J. Cell. Mol. Med. 2013, 17, 672–680. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Oh, K.N.; Yun, H.J.; Jeong, H.G. The flavonoids apigenin and luteolin suppress ultraviolet a-induced matrix metalloproteinase-1 expression via mapks and ap-1-dependent signaling in hacat cells. J. Dermatol. Sci. 2011, 61, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Griffiths, C.E. The use of retinoids in the treatment of photoaging. Dermatol. Ther. 2006, 19, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Madan, K. The role of safranal and saffron stigma extracts in oxidative stress, diseases and photoaging: A systematic review. Heliyon 2021, 7, e06117. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.-L.; Fang, J.-Y.; Chen, M.; Wu, C.-J.; Huang, C.-C.; Hung, C.-F. Chrysin protects epidermal keratinocytes from uva-and uvb-induced damage. J. Agric. Food Chem. 2011, 59, 8391–8400. [Google Scholar] [CrossRef]
- Huang, K.F.; Ma, K.H.; Chang, Y.J.; Lo, L.C.; Jhap, T.Y.; Su, Y.H.; Liu, P.S.; Chueh, S.H. Baicalein inhibits matrix metalloproteinase 1 expression via activation of trpv 1-ca-erk pathway in ultraviolet b–irradiated human dermal fibroblasts. Exp. Dermatol. 2019, 28, 568–575. [Google Scholar] [CrossRef]
- Zhang, J.-A.; Yin, Z.; Ma, L.-W.; Yin, Z.-Q.; Hu, Y.-Y.; Xu, Y.; Wu, D.; Permatasari, F.; Luo, D.; Zhou, B.-R. The protective effect of baicalin against uvb irradiation induced photoaging: An in vitro and in vivo study. PLoS ONE 2014, 9, e99703. [Google Scholar] [CrossRef]
- Min, W.; Liu, X.; Qian, Q.; Lin, B.; Wu, D.; Wang, M.; Ahmad, I.; Yusuf, N.; Luo, D. The effects of baicalin against uva-induced photoaging in skin fibroblasts. Am. J. Chin. Med. 2014, 42, 709–727. [Google Scholar] [CrossRef] [PubMed]
- Min, W.; Lin, X.-F.; Miao, X.; Wang, B.-T.; Yang, Z.-L.; Luo, D. Inhibitory effects of baicalin on ultraviolet b-induced photo-damage in keratinocyte cell line. Am. J. Chin. Med. 2008, 36, 745–760. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.R.; Lin, B.J.; Jin, S.L.; Luo, D. Mitigation of acute ultraviolet b radiation-mediated damages by baicalin in mouse skin. Photodermatol. Photoimmunol. Photomed. 2009, 25, 250–258. [Google Scholar] [CrossRef]
- Sherwani, M.A.; Yang, K.; Jani, A.; Abed, R.A.; Taufique, A.K.; Dosunmu, T.G.; Yusuf, N. Protective effect of baicalin against tlr4-mediated uva-induced skin inflammation. Photochem. Photobiol. 2019, 95, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Cano-Martínez, A.; Bautista-Pérez, R.; Castrejón-Téllez, V.; Carreón-Torres, E.; Pérez-Torres, I.; Díaz-Díaz, E.; Flores-Estrada, J.; Guarner-Lans, V.; Rubio-Ruíz, M.E. Resveratrol and quercetin as regulators of inflammatory and purinergic receptors to attenuate liver damage associated to metabolic syndrome. Int. J. Mol. Sci. 2021, 22, 8939. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Q.; Liang, X.; Xie, J.; Sun, Q. Quercetin reduces inflammation in a rat model of diabetic peripheral neuropathy by regulating the tlr4/myd88/nf-κb signalling pathway. Eur. J. Pharmacol. 2021, 912, 174607. [Google Scholar] [CrossRef]
- Zhao, F.; Dang, Y.; Zhang, R.; Jing, G.; Liang, W.; Li, Z. Apigenin attenuates acrylonitrile-induced neuro-inflammation in rats: Involved of inactivation of the tlr4/nf-κb signaling pathway. Int. Immunopharmacol. 2019, 75, 105697. [Google Scholar] [CrossRef]
- Dong, J.; Xu, O.; Wang, J.; Shan, C.; Ren, X. Luteolin ameliorates inflammation and th1/th2 imbalance via regulating the tlr4/nf-κb pathway in allergic rhinitis rats. Immunopharmacol. Immunotoxicol. 2021, 43, 319–327. [Google Scholar] [CrossRef]
- Maini, S.; Fahlman, B.M.; Krol, E.S. Flavonols protect against uv radiation-induced thymine dimer formation in an artificial skin mimic. J. Pharm. Pharm. Sci. 2015, 18, 600–615. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-H.; Huang, C.-C.; Fang, J.-Y.; Yang, C.; Chan, C.-M.; Wu, N.-L.; Kang, S.-W.; Hung, C.-F. Protective effects of myricetin against ultraviolet-b-induced damage in human keratinocytes. Toxicol. In Vitro 2010, 24, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.K.; Lee, K.W.; Kim, H.Y.; Oh, M.H.; Byun, S.; Lim, S.H.; Heo, Y.-S.; Kang, N.J.; Bode, A.M.; Dong, Z. Myricetin suppresses uvb-induced wrinkle formation and mmp-9 expression by inhibiting raf. Biochem. Pharmacol. 2010, 79, 1455–1461. [Google Scholar] [CrossRef] [Green Version]
- Fitsiou, E.; Pulido, T.; Campisi, J.; Alimirah, F.; Demaria, M. Cellular senescence and the senescence-associated secretory phenotype as drivers of skin photoaging. J. Investig. Dermatol. 2021, 141, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Nan, W.; Ding, L.; Chen, H.; Khan, F.U.; Yu, L.; Sui, X.; Shi, X. Topical use of quercetin-loaded chitosan nanoparticles against ultraviolet b radiation. Front. Pharmacol. 2018, 9, 826. [Google Scholar] [CrossRef]
- Vicentini, F.T.; He, T.; Shao, Y.; Fonseca, M.J.; Verri Jr, W.A.; Fisher, G.J.; Xu, Y. Quercetin inhibits uv irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing nf-κb pathway. J. Dermatol. Sci. 2011, 61, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, R.; Georgetti, S.R.; Verri Jr, W.A.; Dorta, D.J.; dos Santos, A.C.; Fonseca, M.J. Protective effect of topical formulations containing quercetin against uvb-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B Biol. 2006, 84, 21–27. [Google Scholar] [CrossRef]
- Yin, Y.; Li, W.; Son, Y.-O.; Sun, L.; Lu, J.; Kim, D.; Wang, X.; Yao, H.; Wang, L.; Pratheeshkumar, P. Quercitrin protects skin from uvb-induced oxidative damage. Toxicol. Appl. Pharmacol. 2013, 269, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, R.G.; Pinheiro, M.; Neves, A.R. Nanotechnology innovations to enhance the therapeutic efficacy of quercetin. Nanomaterials 2021, 11, 2658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.; Müller, R.; Bégu, S. Quercetin topical application, from conventional dosage forms to nanodosage forms. Eur. J. Pharm. Biopharm. 2016, 108, 41–53. [Google Scholar] [CrossRef]
- Chiang, H.-M.; Chan, S.-Y.; Chu, Y.; Wen, K.-C. Fisetin ameliorated photodamage by suppressing the mitogen-activated protein kinase/matrix metalloproteinase pathway and nuclear factor-κb pathways. J. Agric. Food Chem. 2015, 63, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Lohakul, J.; Chaiprasongsuk, A.; Jeayeng, S.; Saelim, M.; Muanjumpon, P.; Thanachaiphiwat, S.; Tripatara, P.; Soontrapa, K.; Lumlerdkij, N.; Akarasereenont, P. The protective effect of polyherbal formulation, harak formula, on uva-induced photoaging of human dermal fibroblasts and mouse skin via promoting nrf2-regulated antioxidant defense. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Li, M.; Lin, X.-f.; Lu, J.; Zhou, B.-R.; Luo, D. Hesperidin ameliorates uv radiation-induced skin damage by abrogation of oxidative stress and inflammatory in hacat cells. J. Photochem. Photobiol. B Biol. 2016, 165, 240–245. [Google Scholar] [CrossRef]
- Lee, H.J.; Im, A.-R.; Kim, S.-M.; Kang, H.-S.; Lee, J.D.; Chae, S. The flavonoid hesperidin exerts anti-photoaging effect by downregulating matrix metalloproteinase (mmp)-9 expression via mitogen activated protein kinase (mapk)-dependent signaling pathways. BMC Complementary Altern. Med. 2018, 18, 39. [Google Scholar] [CrossRef]
- Jung, S.K.; Ha, S.J.; Jung, C.H.; Kim, Y.T.; Lee, H.K.; Kim, M.O.; Lee, M.H.; Mottamal, M.; Bode, A.M.; Lee, K.W. Naringenin targets erk 2 and suppresses uvb-induced photoaging. J. Cell. Mol. Med. 2016, 20, 909–919. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Silva, T.C.; Caviglione, C.V.; Bottura, C.; Fonseca, M.J.; Vicentini, F.T.; Vignoli, J.A.; Baracat, M.M. Topical formulation containing naringenin: Efficacy against ultraviolet b irradiation-induced skin inflammation and oxidative stress in mice. PLoS ONE 2016, 11, e0146296. [Google Scholar]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [Green Version]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (egcg) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in uv radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Min, W.; Lin, X.-F.; Wu, D.; Xu, Y.; Miao, X. Effect of epigallocatechingallate on ultraviolet b-induced photo-damage in keratinocyte cell line. Am. J. Chin. Med. 2006, 34, 911–922. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Choi, J.-S.; Choi, Y.-J.; Shin, S.-Y.; Kang, S.-W.; Han, S.J.; Kang, Y.-H. (−) Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-b-irradiated human dermal fibroblasts: Involvement of mitogen-activated protein kinase. Food Chem. Toxicol. 2008, 46, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Sevın, A.; Öztaş, P.; Senen, D.; Han, Ü.; Karaman, C.; Tarimci, N.; Kartal, M.; Erdoǧan, B. Effects of polyphenols on skin damage due to ultraviolet a rays: An experimental study on rats. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 650–656. [Google Scholar] [CrossRef]
- Antoniou, C.; Kosmadaki, M.G.; Stratigos, A.J.; Katsambas, A.D. Photoaging. Am. J. Clin. Dermatol. 2010, 11, 95–102. [Google Scholar] [CrossRef]
- Stern, R.S. Treatment of photoaging. N. Engl. J. Med. 2004, 350, 1526–1534. [Google Scholar] [CrossRef]
- Abu Hajleh, M.N.; Abu-Huwaij, R.; AL-Samydai, A.; Al-Halaseh, L.K.; Al-Dujaili, E.A. The revolution of cosmeceuticals delivery by using nanotechnology: A narrative review of advantages and side effects. J. Cosmet. Dermatol. 2021, 20, 3818–3828. [Google Scholar] [CrossRef]
- Gao, X.-H.; Zhang, L.; Wei, H.; Chen, H.-D. Efficacy and safety of innovative cosmeceuticals. Clin. Dermatol. 2008, 26, 367–374. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Mukherjee, P.K.; Kar, A.; Bahadur, S.; Al-Dhabi, N.A.; Duraipandiyan, V. Enhancement of photoprotection potential of catechin loaded nanoemulsion gel against uva induced oxidative stress. J. Photochem. Photobiol. B: Biol. 2016, 160, 318–329. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wu, W.; Chen, H.C.; Fang, H. Genistein protects against uvb-induced senescence-like characteristics in human dermal fibroblast by p66shc down-regulation. J. Dermatol. Sci. 2010, 58, 19–27. [Google Scholar] [CrossRef]
- Sánchez-Marzo, N.; Pérez-Sánchez, A.; Ruiz-Torres, V.; Martínez-Tébar, A.; Castillo, J.; Herranz-López, M.; Barrajón-Catalán, E. Antioxidant and photoprotective activity of apigenin and its potassium salt derivative in human keratinocytes and absorption in caco-2 cell monolayers. Int. J. Mol. Sci. 2019, 20, 2148. [Google Scholar] [CrossRef] [Green Version]
- Esposito, L.; Barbosa, A.I.; Moniz, T.; Costa Lima, S.; Costa, P.; Celia, C.; Reis, S. Design and characterization of sodium alginate and poly (vinyl) alcohol hydrogels for enhanced skin delivery of quercetin. Pharmaceutics 2020, 12, 1149. [Google Scholar] [CrossRef]
- Parashar, P.; Pal, S.; Dwivedi, M.; Saraf, S.A. Augmented therapeutic efficacy of naringenin through microemulsion-loaded sericin gel against uvb-induced photoaging. AAPS PharmSciTech 2020, 21, 215. [Google Scholar] [CrossRef]
- Castañeda-Reyes, E.D.; de Jesús Perea-Flores, M.; Davila-Ortiz, G.; Lee, Y.; de Mejia, E.G. Development, characterization and use of liposomes as amphipathic transporters of bioactive compounds for melanoma treatment and reduction of skin inflammation: A review. Int. J. Nanomed. 2020, 15, 7627. [Google Scholar] [CrossRef]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to uva and uvb radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.J.; Lee, S.-N.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Kwon, S.B.; Kim, M.J. Biological effects of rutin on skin aging. Int. J. Mol. Med. 2016, 38, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Hurtado, P.A.; Garduño-Siciliano, L.; Domínguez-Verano, P.; Balderas-Cordero, D.; Gorgua-Jiménez, G.; Canales-Álvarez, O.; Canales-Martínez, M.M.; Rodríguez-Monroy, M.A. Propolis and its gastroprotective effects on nsaid-induced gastric ulcer disease: A systematic review. Nutrients 2021, 13, 3169. [Google Scholar] [CrossRef]
- Saito, Y.; Tsuruma, K.; Ichihara, K.; Shimazawa, M.; Hara, H. Brazilian green propolis water extract up-regulates the early expression level of ho-1 and accelerates nrf2 after uva irradiation. BMC Complementary Altern. Med. 2015, 15, 421. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Auh, J.-H.; Oh, J.; Hong, S.; Choi, S.; Shin, E.J.; Woo, S.O.; Lim, T.-G.; Byun, S. Propolis suppresses uv-induced photoaging in human skin through directly targeting phosphoinositide 3-kinase. Nutrients 2020, 12, 3790. [Google Scholar] [CrossRef]
- Karapetsas, A.; Voulgaridou, G.-P.; Konialis, M.; Tsochantaridis, I.; Kynigopoulos, S.; Lambropoulou, M.; Stavropoulou, M.-I.; Stathopoulou, K.; Aligiannis, N.; Bozidis, P. Propolis extracts inhibit uv-induced photodamage in human experimental in vitro skin models. Antioxidants 2019, 8, 125. [Google Scholar] [CrossRef] [Green Version]
- Bolfa, P.; Vidrighinescu, R.; Petruta, A.; Dezmirean, D.; Stan, L.; Vlase, L.; Damian, G.; Catoi, C.; Filip, A.; Clichici, S. Photoprotective effects of romanian propolis on skin of mice exposed to uvb irradiation. Food Chem. Toxicol. 2013, 62, 329–342. [Google Scholar] [CrossRef]
- Murase, H.; Shimazawa, M.; Kakino, M.; Ichihara, K.; Tsuruma, K.; Hara, H. The effects of brazilian green propolis against excessive light-induced cell damage in retina and fibroblast cells. Evid. -Based Complementary Altern. Med. 2013, 2013, 238279. [Google Scholar] [CrossRef]
- Ebadi, P.; Fazeli, M. Anti-photoaging potential of propolis extract in uvb-irradiated human dermal fibroblasts through increasing the expression of foxo3a and ngf genes. Biomed. Pharmacother. 2017, 95, 47–54. [Google Scholar] [CrossRef]
- Kim, H.B.; Yoo, B.S. Propolis inhibits uva-induced apoptosis of human keratinocyte hacat cells by scavenging ros. Toxicol. Res. 2016, 32, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Butnariu, M.V.; Giuchici, C.V. The use of some nanoemulsions based on aqueous propolis and lycopene extract in the skin’s protective mechanisms against uva radiation. J. Nanobiotechnology 2011, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Correa, L.; de Carvalho Meirelles, G.; Balestrin, L.; de Souza, P.O.; Moreira, J.C.F.; Schuh, R.S.; Bidone, J.; von Poser, G.L.; Teixeira, H.F. In vitro protective effect of topical nanoemulgels containing brazilian red propolis benzophenones against uv-induced skin damage. Photochem. Photobiol. Sci. 2020, 19, 1460–1469. [Google Scholar] [CrossRef]
- Diniz, D.P.; Lorencini, D.A.; Berretta, A.A.; Cintra, M.A.; Lia, E.N.; Jordão, A.A.; Coelho, E.B. Antioxidant effect of standardized extract of propolis (epp-af®) in healthy volunteers: A “before and after” clinical study. Evid. -Based Complementary Altern. Med. 2020, 2020, 7538232. [Google Scholar] [CrossRef]
- Vijayalakshmi, A.; Ravichandiran, V.; Velraj, M.; Nirmala, S.; Jayakumari, S. Screening of flavonoid “quercetin” from the rhizome of smilax china linn. For anti–psoriatic activity. Asian Pac. J. Trop. Biomed. 2012, 2, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.-H.; Wang, C.-N.; Cheng, H.-H.; Liao, J.-W.; Chen, Y.-T.; Chao, Y.-W.; Jiang, J.L.; Lee, C.-C. Baicalin ameliorates imiquimod-induced psoriasis-like inflammation in mice. Planta Med. 2018, 84, 1110–1117. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Lu, C.; Deng, J.; Yan, Y.; Chen, H.; Wang, Y.; Liang, C.L.; Wei, J.; Han, L. Kaempferol attenuates imiquimod-induced psoriatic skin inflammation in a mouse model. Clin. Exp. Immunol. 2019, 198, 403–415. [Google Scholar] [CrossRef]
- Ogawa, E.; Sato, Y.; Minagawa, A.; Okuyama, R. Pathogenesis of psoriasis and development of treatment. J. Dermatol. 2018, 45, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Jeong, I.; Lee, H.J. Psoriasis skin models as promising tools in psoriasis research. Biomed. J. 2018, 2, 4. [Google Scholar]
- Weng, Z.; Patel, A.B.; Vasiadi, M.; Therianou, A.; Theoharides, T.C. Luteolin inhibits human keratinocyte activation and decreases nf-κb induction that is increased in psoriatic skin. PLoS ONE 2014, 9, e90739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, J.; Zhou, D.; Wang, Y.; Sun, W.; Zhang, C.; Xu, J.; Yang, H.; Zhou, T.; Li, P. Effects of luteolin on treatment of psoriasis by repressing hsp90. Int. Immunopharmacol. 2020, 79, 106070. [Google Scholar] [CrossRef]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-glucoside inhibits il-22/stat3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis. 2016, 7, e2344. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.F.; Ma, K.H.; Liu, P.S.; Chen, B.W.; Chueh, S.H. Baicalein increases keratin 1 and 10 expression in hacat keratinocytes via trpv 4 receptor activation. Exp. Dermatol. 2016, 25, 623–629. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Li, M. Effects of baicalin cream in two mouse models: 2, 4-dinitrofluorobenzene-induced contact hypersensitivity and mouse tail test for psoriasis. Int. J. Clin. Exp. Med. 2015, 8, 2128. [Google Scholar] [PubMed]
- Chang, S.N.; Dey, D.K.; Oh, S.T.; Kong, W.H.; Cho, K.H.; Al-Olayan, E.M.; Hwang, B.S.; Kang, S.C.; Park, J.G. Phorbol 12-myristate 13-acetate induced toxicity study and the role of tangeretin in abrogating hif-1α-nf-κb crosstalk in vitro and in vivo. Int. J. Mol. Sci. 2020, 21, 9261. [Google Scholar] [CrossRef]
- Chen, H.; Lu, C.; Liu, H.; Wang, M.; Zhao, H.; Yan, Y.; Han, L. Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the nf-κb pathway. Int. Immunopharmacol. 2017, 48, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Esnault, S.; Adhami, V.M.; Noll, A.L.; Banang-Mbeumi, S.; Roy, T.; Singh, S.S.; Huang, S.; Kousoulas, K.G.; Mukhtar, H. Fisetin, a 3, 7, 3′, 4′-tetrahydroxyflavone inhibits the pi3k/akt/mtor and mapk pathways and ameliorates psoriasis pathology in 2d and 3d organotypic human inflammatory skin models. Cells 2019, 8, 1089. [Google Scholar] [CrossRef] [Green Version]
- Chlapanidas, T.; Perteghella, S.; Leoni, F.; Faragò, S.; Marazzi, M.; Rossi, D.; Martino, E.; Gaggeri, R.; Collina, S. Tnf-α blocker effect of naringenin-loaded sericin microparticles that are potentially useful in the treatment of psoriasis. Int. J. Mol. Sci. 2014, 15, 13624–13636. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xie, X.; Zhang, L.; Meng, Y.; Li, N.; Wang, M.; Zhai, C.; Liu, Z.; Di, T.; Zhang, L. Hesperidin inhibits keratinocyte proliferation and imiquimod-induced psoriasis-like dermatitis via the irs-1/erk1/2 pathway. Life Sci. 2019, 219, 311–321. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Siddiqui, I.A.; Adhami, V.M.; Esnault, S.; Bharali, D.J.; Babatunde, A.S.; Adame, S.; Massey, R.J.; Wood, G.S.; Longley, B.J. Chitosan-based nanoformulated (−)-epigallocatechin-3-gallate (egcg) modulates human keratinocyte-induced responses and alleviates imiquimod-induced murine psoriasiform dermatitis. Int. J. Nanomed. 2018, 13, 4189. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liu, X.; Mei, L.; Wang, H.; Fang, F. Epigallocatechin-3-gallate (egcg) inhibits imiquimod-induced psoriasis-like inflammation of balb/c mice. BMC Complementary Altern. Med. 2016, 16, 334. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Wei, J.; Lu, C.; Chen, H.; Zhong, X.; Lu, Y.; Li, L.; Huang, H.; Dai, Z.; Han, L. Genistein suppresses psoriasis-related inflammation through a stat3–nf-κb-dependent mechanism in keratinocytes. Int. Immunopharmacol. 2019, 69, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Servidio, C.; Laganà, A.S.; Conforti, F.; Marrelli, M.; Cassano, R. Viscosified solid lipidic nanoparticles based on naringenin and linolenic acid for the release of cyclosporine a on the skin. Molecules 2020, 25, 3535. [Google Scholar] [CrossRef] [PubMed]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.; Müller, R.; Begu, S. Liposomes, lipid nanocapsules and smartcrystals®: A comparative study for an effective quercetin delivery to the skin. Int. J. Pharm. 2018, 542, 176–185. [Google Scholar] [CrossRef]
- El–Gammal, E.; Di Nardo, V.; Daaboul, F.; Tchernev, G.; Wollina, U.; Lotti, J.; Lotti, T. Apitherapy as a new approach in treatment of palmoplantar psoriasis. Open Access Maced. J. Med Sci. 2018, 6, 1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free. Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free. Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Desmet, S.; Morreel, K.; Dauwe, R. Origin and function of structural diversity in the plant specialized metabolome. Plants 2021, 10, 2393. [Google Scholar] [CrossRef]

| Flavonols | Model/UV Radiation | Activities | Ref. |
|---|---|---|---|
| Quercetin, myricetin, and kaempferol | HDFs/UVA | All flavonoids inhibit MMP-1 mRNA levels; presents antioxidant activity | [50] |
| Galangin, kaempferol and quercetin | EpiDermTM/UVA or UVB | All flavonoids reduce MMP-1 and TNF-α secretion; pretreatment reduces the cyclobutane thymine dimers in UVB-irradiation | [70] |
| Myricetin | PHKC/UVB | Decreases MDA level; suppresses H2O2 production and JNK activation | [71] |
| Hairless mice/UVB | Decreases epidermal thickening; inhibits enzyme activity and MMP-9 protein expression; inhibits Raf kinase activity and, consequently, decreases MEK and ERK phosphorylation | [72] | |
| Quercetin and Quercetin nanoparticles | HaCaT cells/UVB | Both decrease NF-κB protein; suppress the IkB-α phosphorylation; reduce the COX-2 protein expression level | [74] |
| C57BL/6 mice/UVB | Decreases IkB-α phosphorylation, COX-2 expression, and PGE2 concentration | ||
| Quercetin | PHKC/UVA and UVB | Reduces NF-κB DNA-binding; inhibits TNF-α, IL-1β, IL-6, and IL-8 expression | [75] |
| Human abdominal skin tissue/UVA and UVB | Inhibits COX-2, MMP-1, and collagen degradation; suppresses NF-κB and AP-1 activation, and JAK2 and PKCδ kinase activity; decreases the Akt, JNK, ERK, and STAT3 phosphorylation | [37] | |
| Hairless mice/UVB | Decreases MPO activity, increases GSH, and suppresses proteinases secretion/activity | [76] | |
| Quercitrin | JB6 cells/UVB | Suppresses apoptosis; reduces C-caspase-3 and C-PARP1 activation; decreases NF-κB activation, DNA damage, 8-OHdG production, γ-H2AX expression, and superoxide radical production; inhibits hydroxyl radical and hydrogen peroxide productions; increases SOD and CAT expressions | [77] |
| Hairless mice/UVB | Decreases apoptosis, C-caspase-3 and C-PARP1 expression; DNA damage; 8-OHdG production; and γ-H2AX expression; increases XPA, SOD and CAT expression and GSH levels | ||
| Fisetin | HDFs/UVB | Inhibits collagen degradation, MMP-9, MMP-3, and MMP-1 expression, COX-2, NO generation, the PGE2 and intracellular ROS, NF-κB translocation into the nucleus and the CREB phosphorylation level; decreases JNK and ERK expression, p38 phosphorylation, and IκB degradation | [81] |
| Propolis/Flavonoids | Model/UV Radiation | Identified Flavonoids | Activities | Ref. |
|---|---|---|---|---|
| Brazil (green propolis) | HDFs/ UVA | N.I. | Inhibits intracellular ROS generation, and ERK and p38 phosphorylation level | [109] |
| N.I. | Positively modulates early HO-1 expression; induces the rapid translocation of Nrf2 to the nucleus | [105] | ||
| Iran | HDFs/ UVB | N.I. | Raises NGF and FOXO3A gene expression; reduces b-galactosidase activity; presents outstanding antioxidant activity | [110] |
| Korea/apigenin, and quercetin | Catechin, naringenin, apigenin, and quercetin | Propolis inhibits MMP-1 production, mRNA levels, and collagen degradation; suppresses Akt, PDK1, and PI3K activity; apigenin and quercetin inhibits PI3K activity | [106] | |
| Korea | HaCaT cells/ UVA | N.I. | Suppresses apoptosis, C-caspase-3 expression; decreases the loss of mitochondrial membrane potential and ROS production | [111] |
| Greece | HaCaT cells/ UVB; EpiDermTM/ UVB | N.I. | In HaCaT cells, presents antioxidant activity; reduces DNA damage and total protein carbonyl content. In EpiDermTM, decreases MMP-9, MMP-7, MMP-3, and MMP-1 mRNA levels | [107] |
| Romania | Swiss mice/ UVB | Luteolin, kaempferol and apigenin | Reduces MDA and IL-6 levels, C-caspase-3 activation, sunburn cell formation, and CPDs generation; increases GPx activity | [108] |
| Flavonols | Model/Psoriasis Inducer | Activities | Ref. |
|---|---|---|---|
| Quercetin | Albino mice | Increases orthokeratosis; decreases granular layer of the epidermis; presents anti-inflammatory effects | [115] |
| BALB/c mice/imiquimod | Reduces psoriatic symptoms, PASI, temperature of the psoriasis-like lesions, histological damage, IL-17, IL-6, and TNF-α; increases SOD, CAT, and GSH; downregulates the expression of RelB, IKKα, and NIK; upregulates TRAF3 expression | [126] | |
| Fisetin | NHEKs, A431, HaCaT, PBMCs and FTRHSP | Inhibits cells growth of NHEKs, A431, HaCaT; on NHEKs induce TGase activity; increases nuclear expression of AP-1 factor subunits (factor Fos (Fos B, c-Fos, and Fra-1/2) y Jun (JunD, JunB, and c-Jun)); suppresses TNF-α-induced activation of MAPK and PI3K/Akt/mTOR pathway; reduces TNF-α, IL-1β, IL-1α, IL-8, IL-6, and TGF-α; in PBMCs inhibits IL-17A and IFN-γ mRNA accumulation; in FTRHSP suppresses expression of desmoglein-1, TGase-1, filaggrin, involucrin, and KRT10, IL-17A, p-p70S6K, and psoriasin markers | [127] |
| Kaempferol | T cells | Suppresses T cells proliferation; inhibits phosphorylation of p70S6K downstream of the mTOR signaling | [117] |
| BALB/c mice/imiquimod | Reduces psoriatic symptoms, histological damage, PASI, CD3+T cell infiltration, IL-17A+CD4+ or RORγt+CD4+ T cells, mRNA expression of TNF-α, IL-6, and IL-17A; inhibits p-NF-κB p65 expression; promotes CD4+FoxP3+ Treg generation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Yañez, C.R.; Ruiz-Hurtado, P.A.; Mendoza-Ramos, M.I.; Reyes-Reali, J.; García-Romo, G.S.; Pozo-Molina, G.; Reséndiz-Albor, A.A.; Nieto-Yañez, O.; Méndez-Cruz, A.R.; Méndez-Catalá, C.F.; et al. Flavonoids Present in Propolis in the Battle against Photoaging and Psoriasis. Antioxidants 2021, 10, 2014. https://doi.org/10.3390/antiox10122014
Rivera-Yañez CR, Ruiz-Hurtado PA, Mendoza-Ramos MI, Reyes-Reali J, García-Romo GS, Pozo-Molina G, Reséndiz-Albor AA, Nieto-Yañez O, Méndez-Cruz AR, Méndez-Catalá CF, et al. Flavonoids Present in Propolis in the Battle against Photoaging and Psoriasis. Antioxidants. 2021; 10(12):2014. https://doi.org/10.3390/antiox10122014
Chicago/Turabian StyleRivera-Yañez, Claudia Rebeca, Porfirio Alonso Ruiz-Hurtado, María Isabel Mendoza-Ramos, Julia Reyes-Reali, Gina Stella García-Romo, Glustein Pozo-Molina, Aldo Arturo Reséndiz-Albor, Oscar Nieto-Yañez, Adolfo René Méndez-Cruz, Claudia Fabiola Méndez-Catalá, and et al. 2021. "Flavonoids Present in Propolis in the Battle against Photoaging and Psoriasis" Antioxidants 10, no. 12: 2014. https://doi.org/10.3390/antiox10122014