Low-Intensity Pulsed Ultrasound Attenuates Postoperative Neurocognitive Impairment and Salvages Hippocampal Synaptogenesis in Aged Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Anesthesia/Surgery
2.3. LIPUS on Hippocampus
2.4. Behavioral Test
2.5. Real Time-qPCR
2.6. Immunofluorescent Staining
2.7. Hematoxylin-Eosin (HE) Staining
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
3.1. LIPUS Could Safely Induce Neuronal Activation in Dorsal Hippocampus
3.2. LIPUS Attenuates Hippocampus-Dependent Spatial Reference Learning and Memory Injury after A/S in Aged Mice
3.3. LIPUS Reduces the Neuroinflammation of Hippocampus after A/S in Aged Mice
3.4. LIPUS Reverses the Reduced Synapse-Related Proteins of the Hippocampus after A/S in Aged Mice
3.5. LIPUS Suppresses the Overexpression of Piezo1 after A/S in Aged Mice
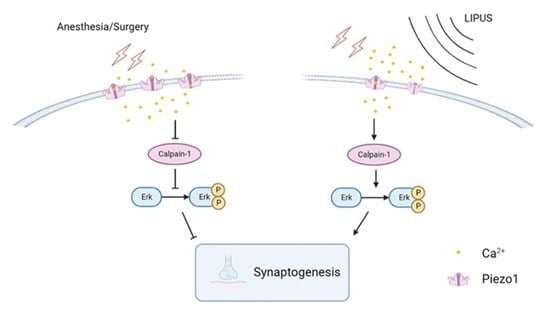
3.6. LIPUS May Improve Synaptic Function through Modulating Piezo1/Calpain/Erk Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shetty, A.K.; Kodali, M.; Upadhya, R.; Madhu, L.N. Emerging Anti-Aging Strategies—Scientific Basis and Efficacy. Aging Dis. 2018, 9, 1165–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmgaard, F.; Vedel, A.G.; Rasmussen, L.S.; Paulson, O.B.; Nilsson, J.C.; Ravn, H.B. The association between postoperative cognitive dysfunction and cerebral oximetry during cardiac surgery: A secondary analysis of a randomised trial. Br. J. Anaesth. 2019, 123, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.N.; Sun, L.; Chen, W.T.; Yang, Y.L.; Wu, Y.M. Effects of edaravone on postoperative cognitive function in elderly patients undergoing hip joint replacement surgery: A randomized controlled trial. Int. J. Surg. 2020, 80, 13–18. [Google Scholar] [CrossRef]
- Rundshagen, I. Postoperative cognitive dysfunction. Dtsch. Arztebl. Int. 2014, 111, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahanna-Gabrielli, E.; Schenning, K.J.; Eriksson, L.I.; Browndyke, J.N.; Wright, C.B.; Evered, L.; Scott, D.A.; Wang, N.Y.; Brown, C.H.; Oh, E.; et al. State of the clinical science of perioperative brain health: Report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. Br. J. Anaesth. 2019, 123, 464–478. [Google Scholar] [CrossRef] [Green Version]
- Saczynski, J.S.; Marcantonio, E.R.; Quach, L.; Fong, T.G.; Gross, A.; Inouye, S.K.; Jones, R.N. Cognitive trajectories after postoperative delirium. N. Engl. J. Med. 2012, 367, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viramontes, O.; Luan Erfe, B.M.; Erfe, J.M.; Brovman, E.Y.; Boehme, J.; Bader, A.M.; Urman, R.D. Cognitive impairment and postoperative outcomes in patients undergoing primary total hip arthroplasty: A systematic review. J. Clin. Anesth. 2019, 56, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Velagapudi, R.; Terrando, N. Neuroinflammation after surgery: From mechanisms to therapeutic targets. Nat. Immunol. 2020, 21, 1319–1326. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. Synaptic Plasticity, Dementia and Alzheimer Disease. CNS Neurol. Disord. Drug Targets 2017, 16, 220–233. [Google Scholar] [CrossRef]
- Mandolesi, G.; Gentile, A.; Musella, A.; Fresegna, D.; De Vito, F.; Bullitta, S.; Sepman, H.; Marfia, G.A.; Centonze, D. Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nat. Rev. Neurol. 2015, 11, 711–724. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, A.K.Y.; Ip, N.Y. Synaptic dysfunction in Alzheimer’s disease: Mechanisms and therapeutic strategies. Pharmacol. Ther. 2019, 195, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Briz, V.; Baudry, M. Calpains: Master Regulators of Synaptic Plasticity. Neuroscientist 2017, 23, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Comprido, D.; Duarte, C.B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 2014, 76, 639–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, M.; Medina, J.H.; Pozzo-Miller, L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn. Mem. 2004, 11, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Mackenzie, S.M.; Storm, D.R. SCOP/PHLPP and its functional role in the brain. Mol. Biosyst. 2010, 6, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Yang, N.; Li, Y.; Li, Y.; Hong, J.; Wang, Q.; Liu, K.; Han, D.; Han, Y.; Mi, X.; et al. Cholecystokinin octapeptide improves hippocampal glutamatergic synaptogenesis and postoperative cognition by inhibiting induction of A1 reactive astrocytes in aged mice. CNS Neurosci. Ther. 2021, 27, 1374–1384. [Google Scholar] [CrossRef]
- Wang, D.S.; Terrando, N.; Orser, B.A. Targeting microglia to mitigate perioperative neurocognitive disorders. Br. J. Anaesth. 2020, 125, 229–232. [Google Scholar] [CrossRef]
- Bystritsky, A.; Korb, A.S.; Douglas, P.K.; Cohen, M.S.; Melega, W.P.; Mulgaonkar, A.P.; DeSalles, A.; Min, B.K.; Yoo, S.S. A review of low-intensity focused ultrasound pulsation. Brain Stimul. 2011, 4, 125–136. [Google Scholar] [CrossRef]
- ter Haar, G. Therapeutic applications of ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 111–129. [Google Scholar] [CrossRef]
- Elias, W.J.; Lipsman, N.; Ondo, W.G.; Ghanouni, P.; Kim, Y.G.; Lee, W.; Schwartz, M.; Hynynen, K.; Lozano, A.M.; Shah, B.B.; et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N. Engl. J. Med. 2016, 375, 730–739. [Google Scholar] [CrossRef]
- Legon, W.; Sato, T.F.; Opitz, A.; Mueller, J.; Barbour, A.; Williams, A.; Tyler, W.J. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci. 2014, 17, 322–329. [Google Scholar] [CrossRef]
- Tufail, Y.; Matyushov, A.; Baldwin, N.; Tauchmann, M.L.; Georges, J.; Yoshihiro, A.; Tillery, S.I.; Tyler, W.J. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron 2010, 66, 681–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, C.Y.; Chiang, P.K.; Tsai, C.W.; Yang, F.Y. Low-Intensity Pulsed Ultrasound Enhances Neurotrophic Factors and Alleviates Neuroinflammation in a Rat Model of Parkinson’s Disease. Cereb. Cortex 2021, 32, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yu, K.; He, B. Transcranial focused ultrasound induces sustained synaptic plasticity in rat hippocampus. Brain Stimul. 2022, 15, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Dell’Italia, J.; Sanguinetti, J.L.; Monti, M.M.; Bystritsky, A.; Reggente, N. Current State of Potential Mechanisms Supporting Low Intensity Focused Ultrasound for Neuromodulation. Front. Hum. Neurosci. 2022, 16, 872639. [Google Scholar] [CrossRef]
- Guo, J.; Gu, D.; Zhao, T.; Zhao, Z.; Xiong, Y.; Sun, M.; Xin, C.; Zhang, Y.; Pei, L.; Sun, J. Trends in Piezo Channel Research Over the Past Decade: A Bibliometric Analysis. Front. Pharmacol. 2021, 12, 668714. [Google Scholar] [CrossRef]
- Fang, X.Z.; Zhou, T.; Xu, J.Q.; Wang, Y.X.; Sun, M.M.; He, Y.J.; Pan, S.W.; Xiong, W.; Peng, Z.K.; Gao, X.H.; et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci. 2021, 11, 13. [Google Scholar] [CrossRef]
- Tang, H.; Zeng, R.; He, E.; Zhang, I.; Ding, C.; Zhang, A. Piezo-Type Mechanosensitive Ion Channel Component 1 (Piezo1): A Promising Therapeutic Target and Its Modulators. J. Med. Chem. 2022, 65, 6441–6453. [Google Scholar] [CrossRef]
- Prieto, M.L.; Firouzi, K.; Khuri-Yakub, B.T.; Maduke, M. Activation of Piezo1 but Not NaV1.2 Channels by Ultrasound at 43 MHz. Ultrasound Med. Biol. 2018, 44, 1217–1232. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Kala, S.; Zhu, J.; Xian, Q.; Qiu, W.; Li, G.; Zhu, T.; Meng, L.; Zhang, R.; et al. The Mechanosensitive Ion Channel Piezo1 Significantly Mediates In Vitro Ultrasonic Stimulation of Neurons. iScience 2019, 21, 448–457. [Google Scholar] [CrossRef]
- Ren, X.; Li, B.; Xu, C.; Zhuang, H.; Lei, T.; Jiang, F.; Zhou, P. High expression of Piezo1 induces senescence in chondrocytes through calcium ions accumulation. Biochem. Biophys. Res. Commun. 2022, 607, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Estevez, M.; Mampay, M.; Boutin, H.; Chaney, A.; Warn, P.; Sharp, A.; Burgess, E.; Moeendarbary, E.; Dev, K.K.; Sheridan, G.K. Infection Augments Expression of Mechanosensing Piezo1 Channels in Amyloid Plaque-Reactive Astrocytes. Front. Aging Neurosci. 2018, 10, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.Y.; Zhang, H.; Ma, T.; Lu, Y.; Xie, H.Y.; Wang, W.; Ma, Y.H.; Li, G.H.; Li, Y.W. Piezo1 mediates neuron oxygen-glucose deprivation/reoxygenation injury via Ca(2+)/calpain signaling. Biochem. Biophys. Res. Commun. 2019, 513, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wang, Y.Y.; Lu, Y.; Feng, L.; Yang, Y.T.; Li, G.H.; Li, C.; Chu, Y.; Wang, W.; Zhang, H. Inhibition of Piezo1/Ca(2+)/calpain signaling in the rat basal forebrain reverses sleep deprivation-induced fear memory impairments. Behav. Brain. Res. 2022, 417, 113594. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, Z.; Liu, T.; Yang, N.; Li, Y.; He, J.; Qian, M.; Kuang, Z.; Zhang, W.; Ni, C.; et al. Prebiotics Regulation of Intestinal Microbiota Attenuates Cognitive Dysfunction Induced by Surgery Stimulation in APP/PS1 Mice. Aging Dis. 2020, 11, 1029–1045. [Google Scholar] [CrossRef]
- He, J.; Liu, T.; Li, Y.; Mi, X.; Han, D.; Yang, N.; Chen, L.; Li, Y.; Hong, J.; Kuang, C.; et al. JNK inhibition alleviates delayed neurocognitive recovery after surgery by limiting microglia pyroptosis. Int. Immunopharmacol. 2021, 99, 107962. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [Green Version]
- Joo, J.Y.; Schaukowitch, K.; Farbiak, L.; Kilaru, G.; Kim, T.K. Stimulus-specific combinatorial functionality of neuronal c-fos enhancers. Nat. Neurosci. 2016, 19, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.R.; Comprido, D.; Duarte, C.B. Regulation of local translation at the synapse by BDNF. Prog. Neurobiol. 2010, 92, 505–516. [Google Scholar] [CrossRef]
- Han, K.; Kim, E. Synaptic adhesion molecules and PSD-95. Prog. Neurobiol. 2008, 84, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Phan, T.; Mansuy, I.M.; Storm, D.R. Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory. Cell 2007, 128, 1219–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folloni, D. Ultrasound neuromodulation of the deep brain. Science 2022, 377, 589. [Google Scholar] [CrossRef] [PubMed]
- Eckenhoff, R.G.; Maze, M.; Xie, Z.; Culley, D.J.; Goodlin, S.J.; Zuo, Z.; Wei, H.; Whittington, R.A.; Terrando, N.; Orser, B.A.; et al. Perioperative Neurocognitive Disorder: State of the Preclinical Science. Anesthesiology 2020, 132, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.T.; Lan, T.H.; Yang, F.Y. Low-Intensity Pulsed Ultrasound Attenuates LPS-Induced Neuroinflammation and Memory Impairment by Modulation of TLR4/NF-kappaB Signaling and CREB/BDNF Expression. Cereb. Cortex 2019, 29, 1430–1438. [Google Scholar] [CrossRef]
- Subramaniyan, S.; Terrando, N. Neuroinflammation and Perioperative Neurocognitive Disorders. Anesth. Analg. 2019, 128, 781–788. [Google Scholar] [CrossRef]
- Wang, B.; Li, S.; Cao, X.; Dou, X.; Li, J.; Wang, L.; Wang, M.; Bi, Y. Blood-brain Barrier Disruption Leads to Postoperative Cognitive Dysfunction. Curr. Neurovasc. Res. 2017, 14, 359–367. [Google Scholar] [CrossRef]
- Hshieh, T.T.; Fong, T.G.; Marcantonio, E.R.; Inouye, S.K. Cholinergic deficiency hypothesis in delirium: A synthesis of current evidence. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Netto, M.B.; de Oliveira Junior, A.N.; Goldim, M.; Mathias, K.; Fileti, M.E.; da Rosa, N.; Laurentino, A.O.; de Farias, B.X.; Costa, A.B.; Rezin, G.T.; et al. Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain Behav. Immun. 2018, 73, 661–669. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Y.; Li, Y.; Han, D.; Liu, T.; Yang, N.; Mi, X.; Hong, J.; Liu, K.; Song, Y.; et al. Inhibition of alpha-Synuclein Accumulation Improves Neuronal Apoptosis and Delayed Postoperative Cognitive Recovery in Aged Mice. Oxidative Med. Cell. Longev. 2021, 2021, 5572899. [Google Scholar] [CrossRef]
- Chen, S.M.; Li, M.; Xie, J.; Li, S.; Xiang, S.S.; Liu, H.Y.; Chen, Z.; Zhang, P.; Kuang, X.; Tang, X.Q. Hydrogen sulfide attenuates postoperative cognitive dysfunction through promoting the pathway of Warburg effect-synaptic plasticity in hippocampus. Toxicol. Appl. Pharmacol. 2020, 409, 115286. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Q.; Wang, L.; Ren, J.; Song, X.; Tian, Y.; Zheng, C.; Yang, J.; Ming, D. Low-Intensity Focused Ultrasound Stimulation Ameliorates Working Memory Dysfunctions in Vascular Dementia Rats via Improving Neuronal Environment. Front. Aging Neurosci. 2022, 14, 814560. [Google Scholar] [CrossRef] [PubMed]
- Leinenga, G.; Götz, J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med. 2015, 7, 278ra233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguchi, K.; Shindo, T.; Ito, K.; Ogata, T.; Kurosawa, R.; Kagaya, Y.; Monma, Y.; Ichijo, S.; Kasukabe, S.; Miyata, S.; et al. Whole-brain low-intensity pulsed ultrasound therapy markedly improves cognitive dysfunctions in mouse models of dementia—Crucial roles of endothelial nitric oxide synthase. Brain Stimul. 2018, 11, 959–973. [Google Scholar] [CrossRef] [Green Version]
- Baudry, M.; Chou, M.M.; Bi, X. Targeting calpain in synaptic plasticity. Expert. Opin. Ther. Targets 2013, 17, 579–592. [Google Scholar] [CrossRef] [Green Version]
- Valtorta, F.; Pennuto, M.; Bonanomi, D.; Benfenati, F. Synaptophysin: Leading actor or walk-on role in synaptic vesicle exocytosis? Bioessays 2004, 26, 445–453. [Google Scholar] [CrossRef]
- Yang, F.Y.; Lu, W.W.; Lin, W.T.; Chang, C.W.; Huang, S.L. Enhancement of Neurotrophic Factors in Astrocyte for Neuroprotective Effects in Brain Disorders Using Low-intensity Pulsed Ultrasound Stimulation. Brain Stimul. 2015, 8, 465–473. [Google Scholar] [CrossRef]







| Gene ID | Protein Name | Forward | Reverse |
|---|---|---|---|
| Tnf | TNF-α | ATGTCTCAGCCTCTTCTCATTC | GCTTGTCACTCGAATTTTGAGA |
| Il1b | IL-1β | CACTACAGGCTCCGAGATGAACAAC | TGTCGTTGCTTGGTTCTCCTTGTAC |
| Il6 | IL-6 | CTCCCAACAGACCTGTCTATAC | CCATTGCACAACTCTTTTCTCA |
| Piezo1 | Piezo1 | GAATGTGATTGGGCAGCGTATGAAC | GAACAGCGTGAGGAACAGACAGTAG |
| Actb | β-actin | TATGCTCTCCCTCACGCCATCC | GTCACGCACGATTTCCCTCTCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Liu, T.; Chang, H.; Li, Z.; Chen, L.; Mi, X.; Xing, H.; Wang, X.; Hong, J.; Liu, K.; et al. Low-Intensity Pulsed Ultrasound Attenuates Postoperative Neurocognitive Impairment and Salvages Hippocampal Synaptogenesis in Aged Mice. Brain Sci. 2023, 13, 657. https://doi.org/10.3390/brainsci13040657
Wang Q, Liu T, Chang H, Li Z, Chen L, Mi X, Xing H, Wang X, Hong J, Liu K, et al. Low-Intensity Pulsed Ultrasound Attenuates Postoperative Neurocognitive Impairment and Salvages Hippocampal Synaptogenesis in Aged Mice. Brain Sciences. 2023; 13(4):657. https://doi.org/10.3390/brainsci13040657
Chicago/Turabian StyleWang, Qian, Taotao Liu, Huixian Chang, Zhengqian Li, Lei Chen, Xinning Mi, Huayi Xing, Xiaoxiao Wang, Jingshu Hong, Kaixi Liu, and et al. 2023. "Low-Intensity Pulsed Ultrasound Attenuates Postoperative Neurocognitive Impairment and Salvages Hippocampal Synaptogenesis in Aged Mice" Brain Sciences 13, no. 4: 657. https://doi.org/10.3390/brainsci13040657