Abnormal Topological Organization of White Matter Structural Networks in Normal Tension Glaucoma Revealed via Diffusion Tensor Tractography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Acquisition Protocol
2.3. Data Processing
2.4. Network Construction
2.5. Network Based Statistics
2.6. Statistical Analysis
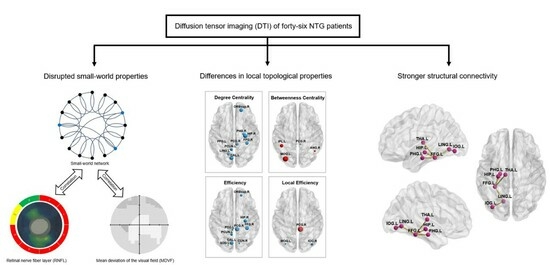
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Between-Group Difference in Global Topological Properties
3.3. Between-Group Difference in Local Topological Properties
3.4. Between-Group Difference in Structural Connectivity Revealed by NBS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gittinger, J.W., Jr. Management of normal tension glaucoma. Surv. Ophthalmol. 2019, 64, 101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Mi, X.S.; So, K.F. Normal tension glaucoma: From the brain to the eye or the inverse? Neural Regen. Res. 2019, 14, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; Zhang, J.; Costantino, F.; De Stefano, N.; Frezzotti, P. Diffuse brain damage in normal tension glaucoma. Hum. Brain Mapp. 2018, 39, 532–541. [Google Scholar] [CrossRef]
- Boucard, C.C.; Hanekamp, S.; Curcic-Blake, B.; Ida, M.; Yoshida, M.; Cornelissen, F.W. Neurodegeneration beyond the primary visual pathways in a population with a high incidence of normal-pressure glaucoma. Ophthalmic Physiol. Opt. 2016, 36, 344–353. [Google Scholar] [CrossRef]
- Afzali, M.; Pieciak, T.; Newman, S.; Garyfallidis, E.; Ozarslan, E.; Cheng, H.; Jones, D.K. The sensitivity of diffusion MRI to microstructural properties and experimental factors. J. Neurosci. Methods 2021, 347, 108951. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.A.; Knott, M.; Heidemann, R.; Michelson, G.; Kober, T.; Dorfler, A.; Engelhorn, T. Investigation of lateral geniculate nucleus volume and diffusion tensor imaging in patients with normal tension glaucoma using 7 tesla magnetic resonance imaging. PLoS ONE 2018, 13, e0198830. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tang, Z.H.; Sun, X.H.; Wu, L.J.; Wang, J.; Zhong, Y.F.; Xiao, Z.B. White Matter Abnormalities and Correlation With Severity in Normal Tension Glaucoma: A Whole Brain Atlas-Based Diffusion Tensor Study. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O. Graph theory methods: Applications in brain networks. Dialogues Clin. Neurosci. 2018, 20, 111–121. [Google Scholar] [CrossRef]
- Wang, J.Q.; Li, T.; Wang, N.L.; Xian, J.F.; He, H.G. Graph theoretical analysis reveals the reorganization of the brain network pattern in primary open angle glaucoma patients. Eur. Radiol. 2016, 26, 3957–3967. [Google Scholar] [CrossRef]
- Minosse, S.; Garaci, F.; Martucci, A.; Lanzafame, S.; Di Giuliano, F.; Picchi, E.; Cesareo, M.; Mancino, R.; Guerrisi, M.; Floris, R.; et al. Disruption of brain network organization in primary open angle glaucoma. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 4338–4341. [Google Scholar]
- Di Cio, F.; Garaci, F.; Minosse, S.; Passamonti, L.; Martucci, A.; Lanzafame, S.; Di Giuliano, F.; Picchi, E.; Cesareo, M.; Guerrisi, M.G.; et al. Reorganization of the structural connectome in primary open angle Glaucoma. NeuroImage Clin. 2020, 28, 102419. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, J.; Fan, L.; Liao, M.; Wang, Y.; Chen, C.; Zhai, T.; Zhang, Y.; Li, L.; Su, L.; et al. White matter structural network disturbances in first-episode, drug-naive adolescents with generalized anxiety disorder. J. Psychiatr. Res. 2020, 130, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhong, S.; Xu, P.; He, Y.; Gong, G. PANDA: A pipeline toolbox for analyzing brain diffusion images. Front. Hum. Neurosci. 2013, 7, 42. [Google Scholar] [CrossRef]
- Basser, P.J.; Pajevic, S.; Pierpaoli, C.; Duda, J.; Aldroubi, A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 2000, 44, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Henje Blom, E.; Flynn, T.; Chen, Y.; Ho, T.C.; Connolly, C.G.; Dumont Walter, R.A.; Yang, T.T.; Xu, D.; Tymofiyeva, O. Test-Retest Reliability of Graph Theoretic Metrics in Adolescent Brains. Brain Connect. 2019, 9, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Shu, N.; Wang, X.; Bi, Q.; Zhao, T.; Han, Y. Disrupted Topologic Efficiency of White Matter Structural Connectome in Individuals with Subjective Cognitive Decline. Radiology 2018, 286, 229–238. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xia, M.; Liao, X.; Evans, A.; He, Y. GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 2015, 9, 386. [Google Scholar] [CrossRef]
- Uehara, T.; Yamasaki, T.; Okamoto, T.; Koike, T.; Kan, S.; Miyauchi, S.; Kira, J.; Tobimatsu, S. Efficiency of a “small-world” brain network depends on consciousness level: A resting-state FMRI study. Cereb. Cortex 2014, 24, 1529–1539. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Shang, S.; Wang, P.; Yin, X.; Krishnan Muthaiah, V.P.; Lu, L.; Chen, Y.C. Functional-structural large-scale brain networks are correlated with neurocognitive impairment in acute mild traumatic brain injury. Quant. Imaging Med. Surg. 2023, 13, 631–644. [Google Scholar] [CrossRef]
- Zalesky, A.; Fornito, A.; Bullmore, E.T. Network-based statistic: Identifying differences in brain networks. NeuroImage 2010, 53, 1197–1207. [Google Scholar] [CrossRef]
- Iturria-Medina, Y.; Sotero, R.C.; Canales-Rodriguez, E.J.; Aleman-Gomez, Y.; Melie-Garcia, L. Studying the human brain anatomical network via diffusion-weighted MRI and Graph Theory. NeuroImage 2008, 40, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Evans, A. Graph theoretical modeling of brain connectivity. Curr. Opin. Neurol. 2010, 23, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.R.; D’Esposito, M. The Segregation and Integration of Distinct Brain Networks and Their Relationship to Cognition. J. Neurosci. 2016, 36, 12083–12094. [Google Scholar] [CrossRef] [PubMed]
- Lalezary, M.; Medeiros, F.A.; Weinreb, R.N.; Bowd, C.; Sample, P.A.; Tavares, I.M.; Tafreshi, A.; Zangwill, L.M. Baseline optical coherence tomography predicts the development of glaucomatous change in glaucoma suspects. Am. J. Ophthalmol. 2006, 142, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Nucci, C.; Strouthidis, N.G.; Khaw, P.T. Neuroprotection and other novel therapies for glaucoma. Curr. Opin. Pharmacol. 2013, 13, 1–4. [Google Scholar] [CrossRef]
- Sporns, O.; Honey, C.J.; Kotter, R. Identification and classification of hubs in brain networks. PLoS ONE 2007, 2, e1049. [Google Scholar] [CrossRef]
- Wurm, M.F.; Caramazza, A. Two ‘what’ pathways for action and object recognition. Trends Cogn. Sci. 2022, 26, 103–116. [Google Scholar] [CrossRef]
- McKendrick, A.M.; Badcock, D.R.; Morgan, W.H. The detection of both global motion and global form is disrupted in glaucoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3693–3701. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Dixon, M.L.; Thiruchselvam, R.; Todd, R.; Christoff, K. Emotion and the prefrontal cortex: An integrative review. Psychol. Bull. 2017, 143, 1033–1081. [Google Scholar] [CrossRef]
- Vikbladh, O.M.; Meager, M.R.; King, J.; Blackmon, K.; Devinsky, O.; Shohamy, D.; Burgess, N.; Daw, N.D. Hippocampal Contributions to Model-Based Planning and Spatial Memory. Neuron 2019, 102, 683–693.e4. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, M.; Zeng, S.; Ma, X.; Yan, J.; Lin, C.; Xu, G.; Li, G.; Yin, Y.; Fu, S.; et al. Abnormal Topology of the Structural Connectome in the Limbic Cortico-Basal-Ganglia Circuit and Default-Mode Network among Primary Insomnia Patients. Front. Neurosci. 2018, 12, 860. [Google Scholar] [CrossRef] [PubMed]
- Frezzotti, P.; Giorgio, A.; Motolese, I.; De Leucio, A.; Iester, M.; Motolese, E.; Federico, A.; De Stefano, N. Structural and functional brain changes beyond visual system in patients with advanced glaucoma. PLoS ONE 2014, 9, e105931. [Google Scholar] [CrossRef] [PubMed]
- Yochim, B.P.; Mueller, A.E.; Kane, K.D.; Kahook, M.Y. Prevalence of cognitive impairment, depression, and anxiety symptoms among older adults with glaucoma. J. Glaucoma 2012, 21, 250–254. [Google Scholar] [CrossRef]
- Cui, Q.N.; Green, D.; Jethi, M.; Driver, T.; Porco, T.C.; Kuo, J.; Lin, S.C.; Stamper, R.L.; Han, Y.; Chiu, C.S.; et al. Individuals with and without normal tension glaucoma exhibit comparable performance on tests of cognitive function. Int. J. Ophthalmol. 2021, 14, 1721–1728. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, Z.; Gao, X.; Huang, W.; Yang, Q.; Li, Z.; Lin, M.; Xiao, H.; Ge, J. Illness uncertainty, anxiety and depression in Chinese patients with glaucoma or cataract. Sci. Rep. 2018, 8, 11671. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, Z.; Wang, K.; Liu, T.; Funahashi, S.; Wu, J.; Zhang, J. Treatment Enhances Betweenness Centrality of Fronto-Parietal Network in Parkinson’s Patients. Front. Comput. Neurosci. 2022, 16, 891384. [Google Scholar] [CrossRef]
- Sack, A.T. Parietal cortex and spatial cognition. Behav. Brain Res. 2009, 202, 153–161. [Google Scholar] [CrossRef]
- Seghier, M.L. The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist 2013, 19, 43–61. [Google Scholar] [CrossRef]
- Sirigu, A.; Duhamel, J.R.; Cohen, L.; Pillon, B.; Dubois, B.; Agid, Y. The mental representation of hand movements after parietal cortex damage. Science 1996, 273, 1564–1568. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Sabel, B.A.; Chen, Z.; Wen, H.; Li, J.; Xie, X.; Yang, D.; Chen, W.; Wang, N.; et al. Structural brain alterations in primary open angle glaucoma: A 3T MRI study. Sci. Rep. 2016, 6, 18969. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Z.; Li, J.; Liu, Z.; Tang, Z.; Xie, X.; Yang, D.; Wang, N.; Tian, J.; Xian, J. Altered amplitude of low-frequency fluctuation in primary open-angle glaucoma: A resting-state FMRI study. Investig. Ophthalmol. Vis. Sci. 2014, 56, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Rauschecker, J.P.; Tian, B.; Korte, M.; Egert, U. Crossmodal changes in the somatosensory vibrissa/barrel system of visually deprived animals. Proc. Natl. Acad. Sci. USA 1992, 89, 5063–5067. [Google Scholar] [CrossRef]
- Rauschecker, J.P. Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci. 1995, 18, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Latora, V.; Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef] [PubMed]
- Frezzotti, P.; Giorgio, A.; Toto, F.; De Leucio, A.; De Stefano, N. Early changes of brain connectivity in primary open angle glaucoma. Hum. Brain Mapp. 2016, 37, 4581–4596. [Google Scholar] [CrossRef]
- Tsapkini, K.; Rapp, B. The orthography-specific functions of the left fusiform gyrus: Evidence of modality and category specificity. Cortex 2010, 46, 185–205. [Google Scholar] [CrossRef]
- Cohen, L.; Lehericy, S.; Chochon, F.; Lemer, C.; Rivaud, S.; Dehaene, S. Language-specific tuning of visual cortex functional properties of the Visual Word Form Area. Brain 2002, 125, 1054–1069. [Google Scholar] [CrossRef]
- Cohen, L.; Dehaene, S.; Naccache, L.; Lehericy, S.; Dehaene-Lambertz, G.; Henaff, M.A.; Michel, F. The visual word form area—Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain 2000, 123, 291–307. [Google Scholar] [CrossRef]
- Del Mauro, G.; Del Maschio, N.; Abutalebi, J. The relationship between reading abilities and the left occipitotemporal sulcus: A dual perspective study. Brain Lang. 2022, 235, 105189. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, C.; Liang, M.; Li, J.; Tian, L.; Zhou, Y.; Qin, W.; Li, K.; Jiang, T. Whole brain functional connectivity in the early blind. Brain 2007, 130, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Turker, S.; Hartwigsen, G. Exploring the neurobiology of reading through non-invasive brain stimulation: A review. Cortex 2021, 141, 497–521. [Google Scholar] [CrossRef] [PubMed]
- Arrington, C.N.; Ossowski, A.E.; Baig, H.; Persichetti, E.; Morris, R. The Impact of Transcranial Magnetic Stimulation on Reading Processes: A Systematic Review. Neuropsychol. Rev. 2023, 33, 255–277. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | NC, n = 19 | NTG, n = 46 | mi-NTG, n = 16 | mo-NTG, n = 13 | se-NTG, n = 17 | p Value | |
|---|---|---|---|---|---|---|---|
| NC vs. NTG | NC vs. NTG Subgroups | ||||||
| Age, y | 49.1 ± 13.5 | 50.0 ± 13.9 | 44.0 ± 11.6 | 51.5 ± 12.7 | 54.5 ± 15.3 | 0.806 | ns |
| Male/female | 10/9 | 26/20 | 9/7 | 7/6 | 10/7 | 0.774 | ns |
| MDVF, dB | |||||||
| Left | −1.2 ± 0.9 | −9.3 ± 6.7 | −3.4 ± 2.5 | −9.1 ± 3.5 | −15.1 ± 6.5 | <0.001 | <0.001 |
| Right | −1.3 ± 0.7 | −10.5 ± 8.4 | −2.5 ± 1.9 | −8.4 ± 4.6 | −19.7 ± 4.8 | <0.001 | <0.001 |
| Mean bilateral eyes | −1.2 ± 0.5 | −9.9 ± 6.7 | −2.9 ± 1.7 | −8.8 ± 1.4 | −17.4 ± 3.3 | <0.001 | <0.001 |
| RNFL thickness, μm | |||||||
| Left | 103.2 ± 9.7 | 78.1 ± 13.1 | 85.3 ± 10.1 | 75.5 ± 11.5 | 73.5 ± 14.5 | <0.001 | <0.001 |
| Right | 102.3 ± 10.5 | 78.2 ± 17.8 | 89.1 ± 13.7 | 81.5 ± 21.0 | 65.3 ± 8.8 | <0.001 | <0.001 |
| Mean bilateral eyes | 102.7 ± 7.6 | 78.2 ± 13.1 | 87.2 ± 9.6 | 78.5 ± 14.2 | 69.4 ± 9.2 | <0.001 | <0.001 |
| Measure | NC | NTG | mi-NTG | mo-NTG | se-NTG | p Value | |||
|---|---|---|---|---|---|---|---|---|---|
| NC vs. NTG | NC vs. mi-NTG | NC vs. mo-NTG | NC vs. se-NTG | ||||||
| Sigma | 1.30 ± 0.09 | 1.24 ± 0.06 | 1.26 ± 0.06 | 1.23 ± 0.06 | 1.22 ± 0.06 | 0.002 ** | 0.227 | 0.018 * | 0.003 ** |
| Lambda | 0.53 ± 0.01 | 0.53 ± 0.01 | 0.53 ± 0.01 | 0.53 ± 0.01 | 0.53 ± 0.01 | 0.054 | 0.976 | 0.102 | 0.057 |
| Gamma | 1.43 ± 0.11 | 1.40 ± 0.09 | 1.34 ± 0.09 | 1.33 ± 0.08 | 1.36 ± 0.09 | 0.007 ** | 0.546 | 0.038 * | 0.008 ** |
| Lp | 1.28 ± 0.04 | 1.30 ± 0.05 | 1.28 ± 0.04 | 1.30 ± 0.07 | 1.32 ± 0.04 | 0.168 | 0.998 | 0.683 | 0.036 * |
| Cp | 0.22 ± 0.01 | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.132 | 0.369 | 0.534 | 0.628 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Guo, L.; Wang, R.; Wang, Y.; Duan, F.; Zhan, Y.; Cheng, J.; Sun, X.; Tang, Z. Abnormal Topological Organization of White Matter Structural Networks in Normal Tension Glaucoma Revealed via Diffusion Tensor Tractography. Brain Sci. 2023, 13, 1597. https://doi.org/10.3390/brainsci13111597
Wang Y, Guo L, Wang R, Wang Y, Duan F, Zhan Y, Cheng J, Sun X, Tang Z. Abnormal Topological Organization of White Matter Structural Networks in Normal Tension Glaucoma Revealed via Diffusion Tensor Tractography. Brain Sciences. 2023; 13(11):1597. https://doi.org/10.3390/brainsci13111597
Chicago/Turabian StyleWang, Yin, Linying Guo, Rong Wang, Yuzhe Wang, Fei Duan, Yang Zhan, Jingfeng Cheng, Xinghuai Sun, and Zuohua Tang. 2023. "Abnormal Topological Organization of White Matter Structural Networks in Normal Tension Glaucoma Revealed via Diffusion Tensor Tractography" Brain Sciences 13, no. 11: 1597. https://doi.org/10.3390/brainsci13111597