Smo-Shh Agonist Purmorphamine Prevents Neurobehavioral and Neurochemical Defects in 8-OH-DPAT-Induced Experimental Model of Obsessive-Compulsive Disorder
Abstract
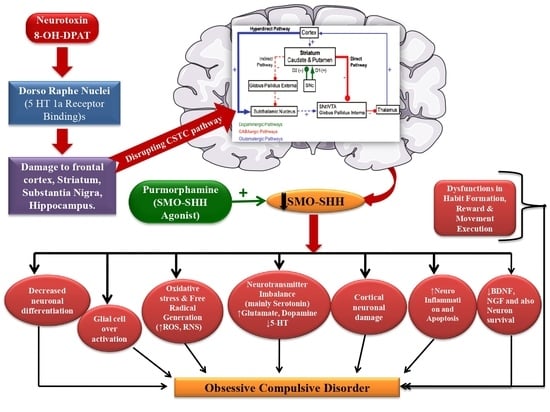
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Chemicals and Drugs
2.3. Experimental Protocol Schedule
2.4. Experimental Animal Model of 8-OH-DPAT-Induced OCD in Adult Rats
2.5. Parameters Assessed
Measurement of Body Weight
2.6. Behavioral Parameters
2.6.1. Compulsive Checking Behavior (CCB)
2.6.2. Forced-Swim Test (FST)
2.6.3. Marble-Burying Behavior (MBB)
2.6.4. Spontaneous Alternation Behavior (SAB)
2.6.5. Signal-Attenuation Model
2.7. Neurochemical Alterations Evaluation and Collection and Preparation of Biological Sample
2.7.1. Brain-Homogenate Preparation
2.7.2. Blood Plasma Collection
2.7.3. CSF Collection
2.7.4. Measurement of Smo-Shh levels
2.8. Measurement of Apoptotic Markers
2.8.1. Measurement of Caspase-3
2.8.2. Measurement of Bax and Bcl-2 Level
2.9. Evaluation of Neuroinflammatory Cytokines
2.9.1. Estimation of TNF-α Level
2.9.2. Estimation of IL-1β Levels
2.10. Evaluation of Neurotransmitters
2.10.1. Serotonin Levels
2.10.2. Glutamate Levels
2.10.3. Dopamine (DOPA) Levels
2.11. Evaluation of Oxidative-Stress Markers
2.11.1. Acetyl Cholinesterase (AChE) Levels
2.11.2. Lactate Dehydrogenase (LDH) Levels
2.11.3. Superoxide Dismutase (SOD) Levels
2.11.4. Reduced-Glutathione (GSH) Levels
2.11.5. Nitrite Levels
2.11.6. Malondialdehyde (MDA) Levels
2.12. Statistical Analysis
3. Results
3.1. Effect of Purmorphamineon Weight Variations in 8-OH OCD
Improvement in Body Weight after Purmorphamine Treatment
3.2. Effect of Purmorphaminein the Treatment of Neurobehavioral Abnormalities in 8-OH-DPAT-Induced Experimental Model of OCD in Adult Rats
3.2.1. Improved Compulsive Checking Behavior in the Open Field after Purmorphamine Treatment
- a.
- Decrease frequency of checking after purmorphamine treatment
- b.
- Increased length of the check after purmorphamine treatment
- c.
- Increase recurrence time of checking after purmorphamine treatment
- d.
- Increase the number of stops before returning to key locale after purmorphamine treatment
3.2.2. Decreased Depression-Like Behavior after Purmorphamine Treatment
3.2.3. Decreased Marble-Burying Behavior after Purmorphamine Treatment
3.2.4. Decreased Spontaneous-Alternation-Behavior (SAB) Score after Purmorphamine Treatment
3.2.5. Reduction in Excessive Lever-Pressing Behavior after Purmorphamine Treatment
3.3. Effect of Purmorphamineon Neurochemical Changes in 8-OH OCD
3.3.1. Increased Smo-Shh Level after Purmorphamine Treatment
3.3.2. Decreased Apoptotic Markers Level after Purmorphamine Treatment
3.3.3. Decreased Inflammatory Cytokines Level after Purmorphamine Treatment
3.3.4. Restoration of Neurotransmitters Level after Purmorphamine Treatment
3.3.5. Decreased Oxidative-Stress-Marker Levelsafter Purmorphamine Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TF’s | Transcriptional Factors |
| BBB | Blood Brain Barrier |
| PPA | Propionic Acid |
| 5-HT | Serotonin |
| 8-OH-DPAT | 8-hydroxy-2-(di-n-propylamino) tetralin |
| 8-OH OCD | 8-hydroxy-2-(di-n-propylamino) tetralin treated rats with obsessive-compulsive disorder |
| AChE | Acetyl cholinesterase |
| CCB | Compulsive checking behavior |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| CSTC | Corticostriatal-thalamocortical pathway |
| DA | Dopamine |
| DMSO | Dimethyl sulfoxide |
| FST | Forced Swim test |
| GSH | Glutathione |
| HPLC | High performance liquid chromatography |
| IDRN | Intra dorsal raphe nucleus |
| IL-1β | Interleukin-1β |
| LDH | Lactate dehydrogenase |
| MBB | Marble burying behavior |
| MDA | Malondialdehyde |
| OCD | Obsessive Compulsive disorder |
| PD | Parkinson’s disease |
| PUR | Purmorphamine |
| ROS | Reactive Oxidative species |
| SAB | Spontaneous alternation behavior |
| SHH | Sonic Hedgehog |
| SMO | Smoothened |
| SOD | Superoxide dismutase |
| SOD | Superoxide dismutase |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
| TNF-α | Tumour necrosis factor |
| v/v | volume/volume |
References
- Stein, D.J.; Costa, D.L.; Lochner, C.; Miguel, E.C.; Reddy, Y.J.; Shavitt, R.G.; van den Heuvel, O.A.; Simpson, H.B. Obsessive–compulsive disorder. Nat. Rev. Dis. Primers 2019, 5, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruscio, A.M.; Stein, D.J.; Chiu, W.T.; Kessler, R.C. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol. Psychiatry 2010, 15, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Hazari, N.; Narayanaswamy, J.C.; Venkatasubramanian, G. Neuroimaging findings in obsessive–compulsive disorder: A narrative review to elucidate neurobiological underpinnings. Indian J. Psychiatry 2019, 61 (Suppl. 1), S9. [Google Scholar] [PubMed]
- Viol, K.; Schiepek, G.; Kronbichler, M.; Hartl, A.; Grafetstätter, C.; Strasser, P.; Kastinger, A.; Schöller, H.; Reiter, E.M.; Said-Yürekli, S.; et al. Multi-level assessment of obsessive-compulsive disorder (OCD) reveals relations between neural and neurochemical levels. BMC Psychiatry 2020, 20, 559. [Google Scholar] [CrossRef] [PubMed]
- Westenberg, H.G.M.; Fineberg, N.A.; Denys, D. Neurobiology of Obsessive-Compulsive Disorder: Serotonin and Beyond. CNS Spectrums. 2007, 12, 14–27. [Google Scholar] [CrossRef]
- Denys, D.; Zohar, J.; Westenberg, H.G. The role of dopamine in obsessive-compulsive disorder: Preclinical and clinical evidence. J Clin Psychiatry 2004, 65 (Suppl. 14), 11–17. [Google Scholar]
- Koo, M.S.; Kim, E.J.; Roh, D.; Kim, C.H. Role of dopamine in the pathophysiology and treatment of obsessive–compulsive disorder. Expert Rev. Neurother. 2010, 10, 275–290. [Google Scholar] [CrossRef]
- Pittenger, C.; Bloch, M.H.; Williams, K. Glutamate abnormalities in obsessive compulsive disorder: Neurobiology, pathophysiology, and treatment. Pharmacol. Ther. 2011, 132, 314–332. [Google Scholar] [CrossRef] [Green Version]
- Furtado, M.; Katzman, M.A. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015, 229, 37–48. [Google Scholar] [CrossRef]
- Gerentes, M.; Pelissolo, A.; Rajagopal, K.; Tamouza, R.; Hamdani, N. Obsessive-compulsive disorder: Autoimmunity and neuroinflammation. Curr. Psychiatry Rep. 2019, 21, 78. [Google Scholar] [CrossRef]
- Aouizerate, B.; Guehl, D.; Cuny, E.; Rougier, A.; Bioulac, B.; Tignol, J.; Burbaud, P. Pathophysiology of obsessive–compulsive disorder: A necessary link between phenomenology, neuropsychology, imagery and physiology. Prog. Neurobiol. 2004, 72, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Baldwin, D.; Abelli, M.; Bolea-Alamanac, B.; Bourin, M.; Chamberlain, S.R.; Cinosi, E.; Davies, S.; Domschke, K.; Fineberg, N.; et al. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World J. Biol. Psychiatry 2017, 18, 162–214. [Google Scholar] [CrossRef] [PubMed]
- Pigott, T.A.; Seay, S.M. A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorder. J. Clin. Psychiatry 1999, 60, 101–106. [Google Scholar] [CrossRef]
- Alonso, P.; López-Solà, C.; Real, E.; Segalàs, C.; Menchón, J.M. Animal models of obsessive–compulsive disorder: Utility and limitations. Neuropsychiatr. Dis. Treat. 2015, 11, 1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadin, E.; Friedman, E.; Bridger, W.H. Spontaneous alternation behavior: An animal model for obsessive-compulsive disorder? Pharmacol. Biochem. Behav. 1991, 40, 311–315. [Google Scholar] [CrossRef]
- Arora, T.; Bhowmik, M.; Khanam, R.; Vohora, D. Oxcarbazepine and fluoxetine protect against mouse models of obsessive compulsive disorder through modulation of cortical serotonin and CREB pathway. Behav. Brain Res. 2013, 247, 146–152. [Google Scholar] [CrossRef]
- Matsushita, M.; Egashira, N.; Harada, S.; Okuno, R.; Mishima, K.; Iwasaki, K.; Nishimura, R.; Fujiwara, M. Perospirone, a novel antipsychotic drug, inhibits marble-burying behavior via 5-HT1A receptor in mice: Implications for obsessive-compulsive disorder. J. Pharmacol. Sci. 2005, 99, 154–159. [Google Scholar] [CrossRef]
- Monti, J.M.; Jantos, H.; Silveira, R.; Reyes-Parada, M.; Scorza, C.; Prunell, G. Depletion of brain serotonin by 5, 7-DHT: Effects on the 8-OH-DPAT-induced changes of sleep and waking in the rat. Psychopharmacology 1994, 115, 273–277. [Google Scholar] [CrossRef]
- Shibata, S.; Tsuneyoshi, A.; Hamada, T.; Tominaga, K.; Watanabe, S. Phase-resetting efect of 8-OH-DPAT, a serotonin1A receptor agonist, on the circadian rhythm of firing rate in the rat suprachiasmatic nuclei in vitro. Brain Res. 1992, 582, 353–356. [Google Scholar] [CrossRef]
- Kim, M.; Kwak, S.; Yoon, Y.B.; Kwak, Y.B.; Kim, T.; Cho, K.I.; Lee, T.Y.; Kwon, J.S. Functional connectivity of the raphe nucleus as a predictor of the response to selective serotonin reuptake inhibitors in obsessive-compulsive disorder. Neuropsychopharmacology 2019, 44, 2073–2081. [Google Scholar] [CrossRef]
- Bernstein, G.A.; Cullen, K.R.; Harris, E.C.; Conelea, C.A.; Zagoloff, A.D.; Carstedt, P.A.; Lee, S.S.; Mueller, B.A. Sertraline effects on striatal resting-state functional connectivity in youth with obsessive-compulsive disorder: A pilot study. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B.; DeVane, C.L.; Pollock, B.G. Newer antidepressants and the cytochrome P450 system. Am. J. Psychiatry 1996, 153, 311–320. [Google Scholar] [PubMed]
- McMahon, A.P.; Ingham, P.W.; Tabin, C.J. Developmental roles and clinical significance of hedgehog signaling. Curr. Top. Dev. Biol. 2003, 53, 1–114. [Google Scholar] [PubMed]
- Placzek, M.; Briscoe, J. Sonic hedgehog in vertebrate neural tube development. Int. J. Dev. Biol. 2018, 62, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ingham, P.W.; McMahon, A.P. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001, 15, 3059–3087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marigo, V.; Tabin, C.J. Regulation of patched by sonic hedgehog in the developing neural tube. Proc. Natl. Acad. Sci. USA 1996, 93, 9346–9351. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Liu, Y.; Yu, Y.; Qi, S.; Liu, Y. Shh signaling guides spatial pathfinding of raphespinal tract axons by multidirectional repulsion. Cell Res. 2012, 22, 697–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonnière, L.; Bernard, M.; et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanizadeh, A. Malondialdehyde, Bcl-2, superoxide dismutase and glutathione peroxidase may mediate the association of sonic hedgehog protein and oxidative stress in autism. Neurochem. Res. 2012, 37, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, I.; Novikova, I.; Augé, J.; Audollent, S.; Esnault, D.; Encha-Razavi, F.; Lazjuk, G.; Attié-Bitach, T.; Vekemans, M. Expression of the sonic hedgehog gene in human embryos with neural tube defects. Teratology 2000, 61, 347–354. [Google Scholar] [CrossRef]
- Wu, J.; He, J.; Tian, X.; Zhong, J.; Li, H.; Sun, X. Activation of the hedgehog pathway promotes recovery of neurological function after traumatic brain injury by protecting the neurovascular unit. Transl. Stroke Res. 2020, 11, 720–733. [Google Scholar] [CrossRef]
- Tayyab, M.; Shahi, M.H.; Farheen, S.; PM, M.M.; Khanam, N.; Hossain, M.M. Exploring the potential role of sonic hedgehog cell signalling pathway in antidepressant effects of nicotine in chronic unpredictable mild stress rat model. Heliyon 2019, 5, e01600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahi, S.; Mehan, S. Understanding abnormal SMO-SHH signaling in autism spectrum disorder: Potential drug target and therapeutic goals. Cell. Mol. Neurobiol. 2020, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Ma, S.; Jia, C.; Su, Y.; Yang, S.; Zhou, K.; Liu, Y.; Cheng, J.; Lu, D.; Fan, L.; et al. Sonic hedgehog is a regulator of extracellular glutamate levels and epilepsy. EMBO Rep. 2016, 17, 682–694. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Chung, Y.C.; Bok, E.; Lee, H.; Huh, S.H.; Lee, J.E.; Jin, B.K.; Ko, H.W. Injury-stimulated Sonic hedgehog expression in microglia contributes to neuroinflammatory response in the MPTP model of Parkinson’s disease. Biochem. Biophys. Res. Commun. 2017, 482, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhou, C.; Wang, L.; Zhu, R.; Zhong, L.; Wan, D.; Wang, Q. Circular RNA SMO sponges miR338-3p to promote the growth of glioma by enhancing the expression of SMO. Aging 2019, 11, 12345–12360. [Google Scholar] [CrossRef] [PubMed]
- Tamayo-Orrego, L.; Charron, F. Recent advances in Shh medulloblastoma progression: Tumor suppressor mechanisms and the tumor microenvironment. F1000Research 2019, 8, 1823. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, J.; Bond, M.C.; Chen, M.; Ren, X.R.; Lyerly, H.K.; Barak, L.S.; Chen, W. Identification of select glucocorticoids as Smoothened agonists: Potential utility for regenerative medicine. Proc. Natl. Acad. Sci. USA 2010, 107, 9323–9328. [Google Scholar] [CrossRef] [Green Version]
- Stanton, B.Z.; Peng, L.F. Small-molecule modulators of the Sonic Hedgehog signaling pathway. Mol. Biosyst. 2010, 6, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Chen, J.K. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat. Chem. Biol. 2006, 2, 29–30. [Google Scholar] [CrossRef]
- Rahi, S.; Gupta, R.; Sharma, A.; Mehan, S. Smo-Shh signaling activator purmorphamine ameliorates neurobehavioral, molecular, and morphological alterations in an intracerebroventricular propionic acid-induced experimental model of autism. Hum. Exp. Toxicol. 2021, 40, 1880–1898. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Bai, X.; Ma, W.; Xin, D.; Chu, X.; Yuan, H.; Qiu, J.; Ke, H.; Yin, S.; Chen, W.; et al. Purmorphamine attenuates neuro-inflammation and synaptic impairments after hypoxic-ischemic injury in neonatal mice via Shh signaling. Front. Pharmacol. 2020, 11, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chechneva, O.V.; Mayrhofer, F.; Daugherty, D.J.; Krishnamurty, R.G.; Bannerman, P.; Pleasure, D.E.; Deng, W. A Smoothened receptor agonist is neuroprotective and promotes regeneration after ischemic brain injury. Cell Death Dis. 2014, 5, e1481. [Google Scholar] [CrossRef] [Green Version]
- Vicario, N.; Bernstock, J.D.; Spitale, F.M.; Giallongo, C.; Giunta, M.A.; Li Volti, G.; Gulisano, M.; Leanza, G.; Tibullo, D.; Parenti, R.; et al. Clobetasol modulates adult neural stem cell growth via canonical hedgehog pathway activation. Int. J. Mol. Sci. 2019, 20, 1991. [Google Scholar] [CrossRef] [Green Version]
- Peterson, R.; Turnbull, J. Sonic hedgehog is cytoprotective against oxidative challenge in a cellular model of amyotrophic lateral sclerosis. J. Mol. Neurosci. 2012, 47, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Wang, G.L.; Raymond, C.; Deng, X.H.; Zhu, X.L.; Wang, D.I.; Hong, L.P. Activation of Sonic hedgehog signal by Purmorphamine, in a mouse model of Parkinson’s disease, protects dopaminergic neurons and attenuates inflammatory response by mediating PI3K/AKt signaling pathway. Mol. Med. Rep. 2017, 16, 1269–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, S.; Bai, X.; Xin, D.; Li, T.; Chu, X.; Ke, H.; Han, M.; Chen, W.; Li, X.; Wang, Z. Neuroprotective effects of the sonic hedgehog signaling pathway in ischemic injury through promotion of synaptic and neuronal health. Neural Plast. 2020, 2020, 8815195. [Google Scholar] [CrossRef] [PubMed]
- Li, P.J.; Guo, Y.Q.; Ding, P.Y.; Liu, R.B.; Deng, F.; Feng, X.X.; Yan, W.J. Neuroprotective effects of a Smoothened receptor agonist against postoperative cognitive dysfunction by promoting autophagy in the dentate gyrus of aged rats. Neurol. Res. 2019, 41, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Tatebayashi, T.; Nagase, K.; Kojima, M.; Imanishi, T. Chronic treatment with fluvoxamine desensitizes 5-HT2C receptor-mediated hypolocomotion in rats. Pharmacol. Biochem. Behav. 2004, 78, 683–689. [Google Scholar] [CrossRef]
- Khera, H.; Awasthi, A.; Mehan, S. Myocardial preconditioning potential of hedgehog activator purmorphamine (smoothened receptor agonist) against ischemia-reperfusion in deoxycortisone acetate salt-induced hypertensive rat hearts. J. Pharmacol. Pharmacother. 2019, 10, 47. [Google Scholar]
- Verma, L.; Sakir, M.; Singh, N.; Mehra, R.; Mehan, S. Development of phase change solutions for ophthalmic drug delivery based on ion activated and pH induced polymers. Int. J. Pharm. Prof. Res. 2010, 1, 127–134. [Google Scholar]
- Celada, P.; Puig, M.V.; Casanovas, J.M.; Guillazo, G.; Artigas, F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABAA, and glutamate receptors. J. Neurosci. 2001, 21, 9917–9929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milton, L.K.; Mirabella, P.N.; Greaves, E.; Spanswick, D.C.; van den Buuse, M.; Oldfield, B.J.; Foldi, C.J. Suppression of corticostriatal circuit activity improves cognitive flexibility and prevents body weight loss in activity-based anorexia in rats. Biol. Psychiatry 2021, 90, 819–828. [Google Scholar] [CrossRef]
- Dvorkin, A.; Silva, C.; McMurran, T.; Bisnaire, L.; Foster, J.; Szechtman, H. Features of compulsive checking behavior mediated by nucleus accumbens and orbital frontal cortex. Eur. J. Neurosci. 2010, 32, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.H.; Dvorkin-Gheva, A.; Szechtman, H. Quinpirole and 8-OH-DPAT induce compulsive checking behavior in male rats by acting on different functional parts of an OCD neurocircuit. Behav. Pharmacol. 2013, 24, 65–73. [Google Scholar] [CrossRef]
- Tiwari, A.; Khera, R.; Rahi, S.; Mehan, S.; Makeen, H.A.; Khormi, Y.H.; Rehman, M.U.; Khan, A. Neuroprotective effect of α-mangostin in the ameliorating propionic acid-induced experimental model of autism in Wistar rats. Brain Sci. 2021, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Rohbani, K.; Sabzevari, S.; Sadat-Shirazi, M.S.; Zadeh-Tehrani, S.N.; Ashabi, G.; Khalifeh, S.; Ale-Ebrahim, M.; Zarrindast, M.R. Parental morphine exposure affects repetitive grooming actions and marble burying behavior in the offspring: Potential relevance for obsessive-compulsive like behavior. Eur. J. Pharmacol. 2019, 865, 172757. [Google Scholar] [CrossRef] [PubMed]
- Albelda, N.; Bar-On, N.; Joel, D. The role of NMDA receptors in the signal attenuation rat model of obsessive–compulsive disorder. Psychopharmacology 2010, 210, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Joel, D.; Avisar, A. Excessive lever pressing following post-training signal attenuation in rats: A possible animal model of obsessive compulsive disorder? Behav. Brain Res. 2001, 123, 77–87. [Google Scholar] [CrossRef]
- Goltseker, K.; Yankelevitch-Yahav, R.; Albelda, N.S.; Joel, D. Signal Attenuation as a Rat Model of Obsessive Compulsive Disorder. J. Vis. Exp. 2015, 95, e52287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Sharma, N.; Khera, R.; Gupta, R.; Mehan, S. Guggulsterone ameliorates ethidium bromide-induced experimental model of multiple sclerosis via restoration of behavioral, molecular, neurochemical and morphological alterations in rat brain. Metab. Brain Dis. 2021, 36, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Khanna, D.; Mehan, S.; Kalra, S. Experimental evidence for the potential of lycopene in the management of scopolamine induced amnesia. RSC Adv. 2015, 5, 72881–72892. [Google Scholar] [CrossRef]
- Van Herck, H.; Baumans, V.; Brandt, C.J.; Hesp, A.P.; Sturkenboom, J.H.; Van Lith, H.A.; Van Tintelen, G.; Beynen, A.C. Orbital sinus blood sampling in rats as performed by different animal technicians: The influence of technique and expertise. Lab. Anim. 1998, 32, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Upadhayay, S.; Mehan, S. Understanding Abnormal c-JNK/p38MAPK Signaling Overactivation Involved in the Progression of Multiple Sclerosis: Possible Therapeutic Targets and Impact on Neurodegenerative Diseases. Neurotox. Res. 2021, 39, 1630–1650. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Rahi, S.; Mehan, S. Neuroprotective potential of solanesol in intracerebroventricular propionic acid induced experimental model of autism: Insights from behavioral and biochemical evidence. Toxicol. Rep. 2019, 6, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Upadhayay, S.; Shandilya, A.; Sahu, R.; Singh, A.; Rajkhowa, B.; Mehan, S. Neuroprotection by solanesol against ethidium bromide-induced multiple sclerosis-like neurobehavioral, molecular, and neurochemical alterations in experimental rats. Phytomed. Plus 2021, 1, 100051. [Google Scholar] [CrossRef]
- Mehan, S.; Rahi, S.; Tiwari, A.; Kapoor, T.; Rajdev, K.; Sharma, R.; Khera, H.; Kosey, S.; Kukkar, U.; Dudi, R. Adenylate cyclase activator forskolin alleviates intracerebroventricular propionic acid-induced mitochondrial dysfunction of autistic rats. Neural Regen. Res. 2020, 15, 1140. [Google Scholar] [CrossRef]
- Alam, M.M.; Minj, E.; Yadav, R.K.; Mehan, S. Neuroprotective potential of adenyl cyclase/cAMP/CREB and mitochondrial CoQ10 activator in amyotrophic lateral sclerosis rats. Curr. Bioact. Compd. 2021, 17, 53–69. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, P. Neuroprotective activity of curcumin in combination with piperine against quinolinic acid induced neurodegeneration in rats. Pharmacology 2016, 97, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Mehan, S.; Bhalla, S.; Kumar, S.; Alshammari, A.; Alharbi, M.; Sadhu, S.S. Guggulsterone Mediated JAK/STAT and PPAR-Gamma Modulation Prevents Neurobehavioral and Neurochemical Abnormalities in Propionic Acid-Induced Experimental Model of Autism. Molecules 2022, 27, 889. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, P.; Deshmukh, R. Neuroprotective potential of spermidine against rotenone induced Parkinson’s disease in rats. Neurochem. Int. 2018, 116, 104–111. [Google Scholar] [CrossRef]
- Jadaun, K.S.; Mehan, S.; Sharma, A.; Siddiqui, E.M.; Kumar, S.; Alsuhaymi, N. Neuroprotective Effect of Chrysophanol as a PI3K/AKT/mTOR Signaling Inhibitor in an Experimental Model of Autologous Blood-induced Intracerebral Hemorrhage. Curr. Med. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Mehan, S.; Monga, V.; Rani, M.; Dudi, R.; Ghimire, K. Neuroprotective effect of solanesol against 3-nitropropionic acid-induced Huntington’s disease-like behavioral, biochemical, and cellular alterations: Restoration of coenzyme-Q10-mediated mitochondrial dysfunction. Indian J. Pharmacol. 2018, 50, 309. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Parveen, S.; Mehan, S.; Khanna, D.; Kalra, S. Neuroprotective effect of ellagic acid against chronically scopolamine induced Alzheimer’s type memory and cognitive dysfunctions: Possible behavioural and biochemical evidences. Int. J. Preven. Med. Res. 2015, 1, 45–64. [Google Scholar]
- Rajdev, K.; Siddiqui, E.M.; Jadaun, K.S.; Mehan, S. Neuroprotective potential of solanesol in a combined model of intracerebral and intraventricular hemorrhage in rats. IBRO Rep. 2020, 8, 101–114. [Google Scholar] [CrossRef]
- Dudi, R.; Mehan, S. Neuroprotection of brain permeable Forskolin ameliorates behavioral, biochemical and histopatho-logical alterations in rat model of intracerebral hemorrhage. Pharmaspire 2018, 10, 68–86. [Google Scholar]
- Abounoori, M.; Maddah, M.M.; Akbari, E.; Houshmand, G.; Ardeshiri, M.R. The effect of orexin receptor antagonism on quinpirole-induced compulsive-like checking behavior in rats. Neurotox. Res. 2020, 38, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Flores-Vargas, A.; Campos-García-Rojas, C.; Jiménez-Ponce, F. Deep brain stimulation of the orbitofrontal cortex reduces perseverative behavior induced by quinpirole in rats. Rev. Méd. Hosp. Gen. México 2019, 82, 15–21. [Google Scholar] [CrossRef]
- Seibell, P.J.; Demarest, J.; Rhoads, D.E. 5-HT1A receptor activity disrupts spontaneous alternation behavior in rats. Pharmacol. Biochem. Behav. 2003, 74, 559–564. [Google Scholar] [CrossRef]
- Ulloa, R.E.; Nicolini, H.; Fernández-Guasti, A. Sex differences on spontaneous alternation in prepubertal rats: Implications for an animal model of obsessive-compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 687–692. [Google Scholar] [CrossRef]
- Umathe, S.N.; Bhutada, P.S.; Jain, N.S.; Mundhada, Y.R.; Borkar, S.S.; Dhumal, B. Role of nitric oxide in obsessive–compulsive behavior and its involvement in the anti-compulsive effect of paroxetine in mice. Nitric Oxide 2009, 21, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Yatsugi, S.I.; Yamaguchi, T. Effect of YM992, a novel antidepressant with selective serotonin re-uptake inhibitory and 5-HT2A receptor antagonistic activity, on a marble-burying behavior test as an obsessive-compulsive disorder model. Jpn. J. Pharmacol. 2002, 90, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, T.; Popik, P. Attenuation of estrous cycle-dependent marble burying in female rats by acute treatment with progesterone and antidepressants. Psychoneuroendocrinology 2007, 32, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Savy, C.Y.; Fitchett, A.E.; McQuade, R.; Gartside, S.E.; Morris, C.M.; Blain, P.G.; Judge, S.J. Low-level repeated exposure to diazinon and chlorpyrifos decrease anxiety-like behaviour in adult male rats as assessed by marble burying behaviour. Neurotoxicology 2015, 50, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, Y.; Xia, J.; Wang, X. Effects of glutamate-related drugs on anxiety and compulsive behavior in rats with obsessive-compulsive disorder. Int. J. Neurosci. 2020, 130, 551–560. [Google Scholar] [CrossRef]
- Sánchez, C.; Meier, E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Psychopharmacology 1997, 129, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, F.; Khan, M.I.; Zubair, M.; Dehpour, A.R. Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomed. Pharmacother. 2018, 105, 1205–1222. [Google Scholar] [CrossRef] [PubMed]
- Lissemore, J.I.; Sookman, D.; Gravel, P.; Berney, A.; Barsoum, A.; Diksic, M.; Nordahl, T.E.; Pinard, G.; Sibon, I.; Cottraux, J.; et al. Brain serotonin synthesis capacity in obsessive-compulsive disorder: Effects of cognitive behavioral therapy and sertraline. Transl. Psychiatry 2018, 8, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutt, D.J.; Forshall, S.; Bell, C.; Rich, A.; Sandford, J.; Nash, J.; Argyropoulos, S. Mechanisms of action of selective serotonin reuptake inhibitors in the treatment of psychiatric disorders. Eur. Neuropsychopharmacol. 1999, 9, S81–S86. [Google Scholar] [CrossRef]
- Kim, M.; Jung, W.H.; Shim, G.; Kwon, J.S. The effects of selective serotonin reuptake inhibitors on brain functional networks during goal-directed planning in obsessive–compulsive disorder. Sci. Rep. 2020, 10, 20619. [Google Scholar] [CrossRef]
- Pampaloni, I.; Sivakumaran, T.; Hawley, C.J.; Al Allaq, A.; Farrow, J.; Nelson, S.; Fineberg, N.A. High-dose selective serotonin reuptake inhibitors in OCD: A systematic retrospective case notes survey. J. Psychopharmacol. 2010, 24, 1439–1445. [Google Scholar] [CrossRef]
- Woody, E.Z.; Szechtman, H. Adaptation to potential threat: The evolution, neurobiology, and psychopathology of the security motivation system. Neurosci. Biobehav. Rev. 2011, 35, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Beliveau, V.; Svarer, C.; Frokjaer, V.G.; Knudsen, G.M.; Greve, D.N.; Fisher, P.M. Functional connectivity of the dorsal and median raphe nuclei at rest. Neuroimage 2015, 116, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Muscatello, M.R.; Bruno, A.; Pandolfo, G.; Micò, U.; Scimeca, G.; Romeo, V.M.; Santoro, V.; Settineri, S.; Spina, E.; Zoccali, R.A. Effect of aripiprazole augmentation of serotonin reuptake inhibitors or clomipramine in treatment-resistant obsessive-compulsive disorder: A double-blind, placebo-controlled study. J. Clin. Psychopharmacol. 2011, 31, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.F.; Wininger, K.; Peyton, L.; Ho, A.M.; Choi, D.S. Astrocytic glutamate transporter 1 (GLT1) deficient mice exhibit repetitive behaviors. Behav. Brain Res. 2021, 396, 112906. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, T.; Goto, S.; Nishikawa, S.; Ushio, Y. Neuronal cell migration for the developmental formation of the mammalian striatum. Brain Res. Rev. 2003, 41, 1–12. [Google Scholar] [CrossRef]
- Karagüzel, E.Ö.; Arslan, F.C.; Uysal, E.K.; Demir, S.; Aykut, D.S.; Tat, M.; Karahan, S.C. Blood levels of interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha and cognitive functions in patients with obsessive compulsive disorder. Compr. Psychiatry 2019, 89, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Ma, W.; Wang, G.T.; Li, Y.J.; Shen, W.D. Antidepressant, anti-inflammatory, and antioxidant effects of electroacupuncture through sonic hedgehog–signaling pathway in a rat model of poststroke depression. Neuropsychiatr. Dis. Treat. 2019, 15, 1403. [Google Scholar] [CrossRef] [Green Version]
- Hassan, W.; Eduardo Barroso Silva, C.; Mohammadzai, I.U.; Batista Teixeira da Rocha, J.; Landeira-Fernandez, J. Association of oxidative stress to the genesis of anxiety: Implications for possible therapeutic interventions. Curr. Neuropharmacol. 2014, 12, 120–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.Y.; Yao, J.K. Oxidative stress and therapeutic implications in psychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 197–199. [Google Scholar] [CrossRef] [PubMed]







| S.no. | Groups | Smo-Shh Protein Level | ||
|---|---|---|---|---|
| Brain Homogenate (nM/µg Protein) | Blood Plasma (ng/mL) | CSF (ng/mL) | ||
| 1. | Vehicle control | 11.89 ± 0.223 | 5.17 ± 0.030 | 2.49 ± 0.007 |
| 2. | Sham control | 11.65 ± 0.191 | 5.11 ± 0.019 | 2.51 ± 0.009 |
| 3. | PUR10 per se | 11.78 ± 0.187 | 5.12 ± 0.015 | 2.50 ± 0.009 |
| 4. | 8-OH-DPAT | 3.79 ± 0.102 * | 2.73 ± 0.054 * | 1.21 ± 0.011 * |
| 5. | 8-OH-DPAT + PUR5 | 4.78 ± 0.055 # | 3.23 ± 0.017 # | 1.46 ± 0.007 # |
| 6. | 8-OH-DPAT + PUR10 | 6.00 ± 0.070 #$ | 3.83 ± 0.024 #$ | 1.76 ± 0.007 #$ |
| 7. | 8-OH-DPAT + FLX10 | 7.63 ± 0.105 #β | 4.16 ± 0.011 #β | 2.06 ± 0.010 #β |
| 8. | 8-OH-DPAT + PUR10 + FLX10 | 9.05 ± 0.095 #@ | 4.65 ± 0.017 #@ | 2.25 ± 0.007 #@ |
| S. no. | Groups | Apoptotic Markers | |||||
|---|---|---|---|---|---|---|---|
| Caspase-3 | Bax | Bcl-2 | |||||
| Brain Homogenate (nM/mg Protein) | Blood Plasma (ng/mL) | Brain Homogenate (ng/mg Protein) | Blood Plasma (ng/mL) | Brain Homogenate (ng/mg Protein) | Blood Plasma (ng/mL) | ||
| 1. | Vehicle control | 110.80 ± 0.691 | 1.66 ± 0.008 | 7.27 ± 0.071 | 1.15 ± 0.007 | 38.06 ± 0.304 | 9.78 ± 0.010 |
| 2. | Sham control | 110.70 ± 0.559 | 1.67 ± 0.008 | 7.30 ± 0.089 | 1.15 ± 0.007 | 38.12 ± 0.352 | 9.79 ± 0.010 |
| 3. | PUR10 per se | 110.70 ± 0.792 | 1.67 ± 0.010 | 7.37 ± 0.075 | 1.15 ± 0.007 | 38.05 ± 0.390 | 9.79 ± 0.011 |
| 4. | 8-OH-DPAT | 160.90 ± 0.939 * | 6.28 ± 0.006 * | 14.53 ± 0.180 * | 5.68 ± 0.007 * | 24.30 ± 0.346 * | 3.59 ± 0.007 * |
| 5. | 8-OH-DPAT + PUR5 | 151.70 ± 0.581 # | 5.57 ± 0.009 # | 13.47 ± 0.043 # | 4.77 ± 0.007 # | 27.03 ± 0.231 # | 4.43 ± 0.007 # |
| 6. | 8-OH-DPAT + PUR10 | 141.00 ± 0.546 #$ | 4.45 ± 0.011 #$ | 11.89 ± 0.033 #$ | 3.56 ± 0.007 #$ | 29.06 ± 0.120 #$ | 5.64 ± 0.009 #$ |
| 7. | 8-OH-DPAT + FLX10 | 132.70 ± 0.365 | 3.36 ± 0.007 #β | 10.49 ± 0.064 #β | 2.86 ± 0.007 #β | 33.04 ± 0.204 #β | 6.51 ± 0.007 #β |
| 8. | 8-OH-DPAT + PUR10 + FLX10 | 123.20 ± 0.345 #@ | 2.21 ± 0.007 #@ | 9.41 ± 0.027 #@ | 1.92 ± 0.007 #@ | 35.70 ± 0.103 #@ | 7.87 ± 0.007 #@ |
| S. no. | Groups | Cytokine Level | |||
|---|---|---|---|---|---|
| TNF-α | IL-1β | ||||
| Brain Homogenate (pg/mg Protein) | Blood Plasma (ng/mL) | Brain Homogenate (pg/mg Protein) | Blood Plasma (ng/mL) | ||
| 1. | Vehicle control | 35.42 ± 0.242 | 4.49 ± 0.014 | 18.75 ± 0.126 | 14.47 ± 0.007 |
| 2. | Sham control | 35.80 ± 0.278 | 4.50 ± 0.014 | 18.92 ± 0.318 | 14.47 ± 0.010 |
| 3. | PUR10 per se | 35.53 ± 0.213 | 4.49 ± 0.011 | 18.67 ± 0.291 | 14.48 ± 0.007 |
| 4. | 8-OH-DPAT | 76.44 ± 0.725 * | 9.49 ± 0.009 * | 33.22 ± 0.198 * | 66.48 ± 0.008 * |
| 5. | 8-OH-DPAT + PUR5 | 67.40 ± 0.657 # | 8.47 ± 0.006 # | 28.35 ± 0.411 # | 55.39 ± 0.012 # |
| 6. | 8-OH-DPAT + PUR10 | 58.30 ± 0.221 #$ | 7.52 ± 0.009 #$ | 25.78 ± 0.223 #$ | 43.71 ± 0.014 #$ |
| 7. | 8-OH-DPAT + FLX10 | 50.01 ± 0.206 #β | 6.77 ± 0.009 #β | 23.08 ± 0.276 #β | 37.48 ± 0.014 #β |
| 8. | 8-OH-DPAT + PUR10 + FLX10 | 42.05 ± 0.170 #@ | 5.37 ± 0.009 #@ | 21.22 ± 0.279 #@ | 24.95 ± 0.008 #@ |
| S. no. | Groups | Neurotransmitters | ||
|---|---|---|---|---|
| Serotonin (ng/mg Protein) | Glutamate (ng/mg Protein) | Dopamine (ng/mg Protein) | ||
| 1. | Vehicle control | 44.24 ± 0.191 | 114.40 ± 0.208 | 95.42 ± 0.294 |
| 2. | Sham control | 44.26 ± 0.205 | 113.90 ± 0.333 | 95.74 ± 0.337 |
| 3. | PUR10 per se | 44.06 ± 0.197 | 113.90 ± 0.307 | 95.50 ± 0.335 |
| 4. | 8-OH-DPAT | 16.33 ± 0.290 * | 305.80 ± 0.316 * | 31.48 ± 0.394 * |
| 5. | 8-OH-DPAT + PUR5 | 22.34 ± 0.232 # | 214.30 ± 0.260 # | 43.10 ± 0.222 # |
| 6. | 8-OH-DPAT + PUR10 | 27.37 ± 0.223 #$ | 186.60 ± 0.248 #$ | 52.00 ± 0.392 #$ |
| 7. | 8-OH-DPAT + FLX10 | 32.87 ± 0.186 #β | 167.50 ± 0.639 #β | 63.05 ± 0.453 #β |
| 8. | 8-OH-DPAT + PUR10 + FLX10 | 38.23 ± 0.236 #@ | 142.20 ± 0.299 #@ | 74.19 ± 0.448 #@ |
| S.no. | Groups | Oxidative-Stress Markers | |||||
|---|---|---|---|---|---|---|---|
| AChE (µM/mg Protein) | LDH (µM/mg Protein) | SOD (µM/mg Protein) | GSH (µM/mg Protein) | Nitrite (µM/mg Protein) | MDA (nM/mg Protein) | ||
| 1. | Vehicle control | 24.12 ± 0.133 | 117.70 ± 0.366 | 478.00 ± 0.684 | 36.51 ± 0.320 | 7.72 ± 0.216 | 35.39 ± 0.240 |
| 2. | Sham control | 24.26 ± 0.208 | 117.20 ± 0.222 | 478.40 ± 0.546 | 36.64 ± 0.329 | 8.07 ± 0.186 | 36.20 ± 0.342 |
| 3. | PUR10 per se | 24.42 ± 0.194 | 117.90 ± 0.329 | 478.20 ± 0.660 | 36.54 ± 0.352 | 7.91 ± 0.295 | 35.75 ± 0.418 |
| 4. | 8-OH-DPAT | 54.44 ± 0.354 * | 401.90 ± 0.851 * | 305.30 ± 0.646 * | 11.61 ± 0.286 * | 14.18 ± 0.259 * | 76.24 ± 0.269 * |
| 5. | 8-OH-DPAT + PUR5 | 46.46 ± 0.337 # | 315.40 ± 1.042 # | 332.60 ± 0.595 # | 16.20 ± 0.171 # | 12.44 ± 0.169 # | 66.64 ± 0.362 # |
| 6. | 8-OH-DPAT + PUR10 | 40.94 ± 0.226 #$ | 275.70 ± 1.046 #$ | 369.20 ± 0.460 #$ | 22.71 ± 0.342 #$ | 11.40 ± 0.091 #$ | 59.21 ± 0.383 #$ |
| 7. | 8-OH-DPAT + FLX10 | 35.83 ± 0.444 #β | 195.80 ± 1.011 #β | 411.40 ± 0.642 #β | 28.11 ± 0.150 #β | 10.45 ± 0.131 #β | 51.38 ± 0.383 #β |
| 8. | 8-OH-DPAT + PUR10 + FLX10 | 30.05 ± 0.212 #@ | 145.30 ± 1.374 #@ | 446.30 ± 0.534 #@ | 32.45 ± 0.226 #@ | 9.47 ± 0.092 #@ | 43.55 ± 0.370 #@ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, R.; Mehan, S.; Sethi, P.; Prajapati, A.; Alshammari, A.; Alharbi, M.; Al-Mazroua, H.A.; Narula, A.S. Smo-Shh Agonist Purmorphamine Prevents Neurobehavioral and Neurochemical Defects in 8-OH-DPAT-Induced Experimental Model of Obsessive-Compulsive Disorder. Brain Sci. 2022, 12, 342. https://doi.org/10.3390/brainsci12030342
Gupta R, Mehan S, Sethi P, Prajapati A, Alshammari A, Alharbi M, Al-Mazroua HA, Narula AS. Smo-Shh Agonist Purmorphamine Prevents Neurobehavioral and Neurochemical Defects in 8-OH-DPAT-Induced Experimental Model of Obsessive-Compulsive Disorder. Brain Sciences. 2022; 12(3):342. https://doi.org/10.3390/brainsci12030342
Chicago/Turabian StyleGupta, Ria, Sidharth Mehan, Pranshul Sethi, Aradhana Prajapati, Abdulrahman Alshammari, Metab Alharbi, Haneen A. Al-Mazroua, and Acharan S. Narula. 2022. "Smo-Shh Agonist Purmorphamine Prevents Neurobehavioral and Neurochemical Defects in 8-OH-DPAT-Induced Experimental Model of Obsessive-Compulsive Disorder" Brain Sciences 12, no. 3: 342. https://doi.org/10.3390/brainsci12030342