Naturalistic Study of Depression Associated with Parkinson’s Disease in a National Public Neurological Referral Center in Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Instruments for Data Collection
2.3. Statistical Analysis
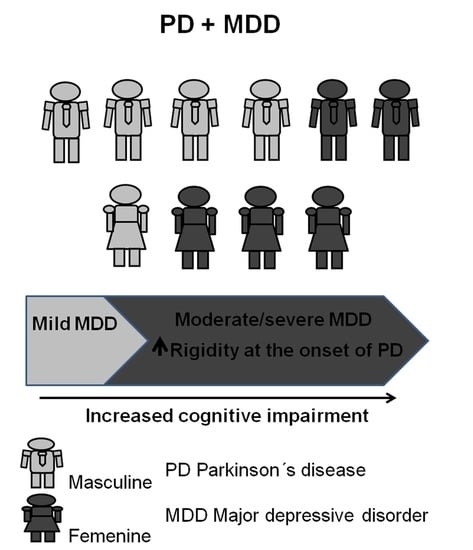
3. Results
3.1. Clinical and Sociodemographic Variables
3.2. Medication Variables
3.3. Comparison between Mild and Moderate–Severe MDD Groups
3.4. Comparison between Sexes in General Sample
3.5. Comparison between Onset with Tremor and Onset with Other Symptoms
3.6. Independent Logistic Regressions for Binary Dependent Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, A.; Gilbert, R.M. Epidemiology of Parkinson Disease. Neurol. Clin. 2016, 34, 955–965. [Google Scholar] [CrossRef]
- GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2021, 17, 939–953, Erratum in Lancet Neurol. 2021, 20, e7. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.I.; Rahman, M.R.; Munira, S.; Jahan, M. Risk Factors of Major Depressive Disorder in Parkinson’s Disease. Bangladesh Med. Res. Counc. Bull. 2018, 44, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Han, J.W.; Ahn, Y.D.; Kim, W.S.; Shin, C.M.; Jeong, S.J.; Song, Y.S.; Bae, Y.J.; Kim, J.M. Psychiatric Manifestation in Patients with Parkinson’s Disease. J. Korean Med. Sci. 2018, 33, e300. [Google Scholar] [CrossRef]
- Kukkle, P.L.; Goyal, V.; Geetha, T.S.; Mridula, K.R.; Kumar, H.; Borgohain, R.; Ramprasad, V.L. Clinical Study of 668 Indian Subjects with Juvenile, Young, and Early Onset Parkinson’s Disease. Can. J. Neurol. Sci. 2022, 49, 93–101, Erratum in Nat. Rev. Neurosci. 2017, 18, 509. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Torbey, E.; Pachana, N.A.; Dissanayaka, N.N. Depression rating scales in Parkinson’s disease: A critical review updating recent literature. J. Affect. Disord. 2015, 184, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Marsili, L.; Rizzo, G.; Colosimo, C. Diagnostic Criteria for Parkinson’s Disease: From James Parkinson to the Concept of Prodromal Disease. Front. Neurol. 2018, 9, 156. [Google Scholar] [CrossRef]
- Williams, J.B.; Kobak, K.A. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br. J. Psychiatry 2008, 192, 52–58. [Google Scholar] [CrossRef]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar] [CrossRef]
- Wikberg, C.; Pettersson, A.; Westman, J.; Björkelund, C.; Petersson, E.L. Patients’ perspectives on the use of the Montgomery-Asberg depression rating scale self-assessment version in primary care. Scand. J. Prim. Health Care 2016, 34, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Chamorro, L.; Luque, A.; Dal-Ré, R.; Badia, X.; Baró, E.; Grupo de Validación en Español de Escalas Psicométricas (GVEEP). Validation of the Spanish versions of the Montgomery-Asberg depression and Hamilton anxiety rating scales. Med. Clin. 2002, 118, 493–499. [Google Scholar] [CrossRef]
- Mitchell, A.J. The Mini-Mental State Examination (MMSE): Update on Its Diagnostic Accuracy and Clinical Utility for Cognitive Disorders. In Cognitive Screening Instruments, 2nd ed.; Larner, A.J., Ed.; Springer: Cham, Switzerland, 2017; pp. 37–48. [Google Scholar] [CrossRef]
- Beaman, S.R.D.; Beaman, P.E.; Garcia-Peña, C.; Villa, M.A.; Heres, J.; Córdova, A.; Jagger, C. Validation of a modified version of the Mini-Mental State Examination (MMSE) in Spanish. Aging Neuropsychol. Cogn. 2004, 11, 1–11. [Google Scholar] [CrossRef]
- Pinto, T.C.C.; Machado, L.; Bulgacov, T.M.; Rodrigues-Júnior, A.L.; Costa, M.L.G.; Ximenes, R.C.C.; Sougey, E.B. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int. Psychogeriatr. 2019, 31, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Navarro, S.G.; Mimenza-Alvarado, A.J.; Palacios-García, A.A.; Samudio-Cruz, A.; Gutiérrez-Gutiérrez, L.A.; Ávila-Funes, J.A. Validity and Reliability of the Spanish Version of the Montreal Cognitive Assessment (MoCA) for the Detection of Cognitive Impairment in Mexico. Rev. Colomb. Psiquiatr. 2018, 47, 237–243. [Google Scholar] [CrossRef]
- Juul, S. An Introduction to STATA for Health Researchers, 1st ed.; STATA Press: College Station, TX, USA, 2006; pp. 127–130. [Google Scholar]
- Sadock, B.J.; Sadock, V.A. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry, 10th ed.; Lippincott Williams & Wilkins Publishers: Philadelphia, PA, USA, 2007; pp. 60–83. [Google Scholar]
- Ray, S.; Agarwal, P. Depression and Anxiety in Parkinson Disease. Clin. Geriatr. Med. 2020, 36, 93–104. [Google Scholar] [CrossRef]
- Riedel, O.; Heuser, I.; Klotsche, J.; Dodel, R.; Wittchen, H.U.; GEPAD Study Group. Occurrence risk and structure of depression in Parkinson disease with and without dementia: Results from the GEPAD Study. J. Geriatr. Psychiatry Neurol. 2010, 23, 27–34. [Google Scholar] [CrossRef]
- Lubomski, M.; Davis, R.L.; Sue, C.M. Depression in Parkinson’s disease: Perspectives from an Australian cohort. J. Affect. Disord. 2020, 277, 1038–1044. [Google Scholar] [CrossRef]
- Cao, Y.; Li, G.; Xue, J.; Zhang, G.; Gao, S.; Huang, Y.; Zhu, A. Depression and Related Factors in Patients with Parkinson’s Disease at High Altitude. Neuropsychiatr. Dis. Treat. 2021, 17, 1353–1362. [Google Scholar] [CrossRef]
- Pir-hayati, M.; Eydivandi, N.; Khodashenas, M.; Fallah, H. Prevalence of Depression and Anxiety and Related Factors in Patients with Parkinson’s Disease: Depression and Anxiety in Parkinson’s Disease. Int. Clin. Neurosci. J. 2021, 8, 85–89. [Google Scholar] [CrossRef]
- Lian, T.H.; Guo, P.; Zuo, L.J.; Hu, Y.; Yu, S.Y.; Liu, L.; Jin, Z.; Yu, Q.J.; Wang, R.D.; Li, L.X.; et al. An Investigation on the Clinical Features and Neurochemical Changes in Parkinson’s Disease with Depression. Front. Psychiatry 2019, 9, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zesiewicz Theresa, A. Parkinson disease. Contin. Lifelong Learn. Neurol. 2019, 25, 896–918. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.H.; Guo, P.; Zuo, L.J.; Hu, Y.; Yu, S.Y.; Yu, Q.J.; Zhang, W. Tremor-dominant in Parkinson disease: The relevance to iron metabolism and inflammation. Front. Neurosci. 2019, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.; Moon, J.K.; Cho, J.W.; Oh, E.; Kim, J.S.; Jang, W.; Park, J. The characteristics of non-motor symptoms in drug-naive Parkinson’s disease: Analysis between tremor dominant and non-tremor dominant subtypes. Mov. Disord. 2014, 11, e0162254. [Google Scholar] [CrossRef] [Green Version]
- Morano, A.; Jiménez-Jiménez, F.J.; Molina, J.A.; Antolín, M.A. Risk-factors for Parkinson’s disease: Case-control study in the province of Caceres, Spain. Acta Neurol. Scand. 1994, 89, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Simon, K.C.; Chen, H.; Schwarzschild, M.; Ascherio, A. Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology 2007, 69, 1688–1695. [Google Scholar] [CrossRef] [Green Version]
- Papapetropoulos, S.; Ellul, J.; Argyriou, A.A.; Chroni, E.; Lekka, N.P. The effect of depression on motor function and disease severity of Parkinson’s disease. Clin. Neurol. Neurosurg. 2006, 108, 465–469. [Google Scholar] [CrossRef]
- Reijnders, J.S.; Ehrt, U.; Lousberg, R.; Aarsland, D.; Leentjens, A.F. The association between motor subtypes and psychopathology in Parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15, 379–382. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, L.; Pan, Y.; Shen, B.; Xu, S.; Hou, Y.; Zhang, X.; Zhang, L. Depression and associated factors in nondemented Chinese patients with Parkinson’s disease. Clin. Neurol. Neurosurg. 2017, 163, 142–148. [Google Scholar] [CrossRef]
- Kincses, P.; Kovács, N.; Karádi, K.; Feldmann, Á.; Dorn, K.; Aschermann, Z.; Komoly, S.; Szolcsányi, T.; Csathó, Á.; Kállai, J. Association of Gait Characteristics and Depression in Patients with Parkinson’s Disease Assessed in Goal-Directed Locomotion Task. Parkinsons Dis. 2017, 2017, 6434689. [Google Scholar] [CrossRef]
- Yapici Eser, H.; Bora, H.A.; Kuruoğlu, A. Depression and Parkinson disease: Prevalence, temporal relationship, and determinants. Turk. J. Med. Sci. 2017, 47, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, K.A.; Valverde, E.M.; Aguilar, D.V.; Gabarain, H.H. Montreal Cognitive Assessment scale in patients with Parkinson Disease with normal scores in the Mini-Mental State Examination. Dement. Neuropsychol. 2019, 13, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Velasco, S.; Llorente-Ayuso, L.; Contador, I.; Bermejo-Pareja, F. Versiones en español del Minimental State Examination (MMSE). Cuestiones para su uso en la practica clinica [Spanish versions of the Minimental State Examination (MMSE). Questions for their use in clinical practice]. Rev. Neurol. 2015, 61, 363–371. [Google Scholar] [PubMed]
- Seppi, K.; Weintraub, D.; Coelho, M.; Perez-Lloret, S.; Fox, S.H.; Katzenschlager, R.; Hametner, E.M.; Poewe, W.; Rascol, O.; Goetz, C.G.; et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson’s disease. Mov. Disord. 2011, 26 (Suppl. S3), S42–S80. [Google Scholar] [CrossRef]
- Seppi, K.; Ray Chaudhuri, K.; Coelho, M.; Fox, S.H.; Katzenschlager, R.; Perez Lloret, S.; Weintraub, D.; Sampaio, C.; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov. Disord. 2019, 34, 180–198. [Google Scholar] [CrossRef] [Green Version]
- Alster, P.; Madetko, N.; Koziorowski, D.; Friedman, A. Progressive Supranuclear Palsy—Parkinsonism Predominant (PSP-P)—A Clinical Challenge at the Boundaries of PSP and Parkinson’s Disease (PD). Front. Neurol. 2020, 11, 180. [Google Scholar] [CrossRef]
- Necpál, J.; Miroslav, B.; Jeleňová, B. “Parkinson’s disease” on the way to progressive supranuclear palsy: A review on PSP-parkinsonism. Neurol. Sci. 2021, 42, 4927–4936. [Google Scholar] [CrossRef]
| Variables | Results |
|---|---|
| Age (years, average ± SD) | 58.49 ± 11.02 |
| Age at onset of PD (years, average ± SD) | 50.66 ± 11.86 |
| Years of PD evolution (average ± SD) | 7.83 ± 5.33 |
| UPDRS III (average ± SD) | 33.67 ± 5.67 |
| MADRS (average ± SD) | 21.33 ± 5.49 |
| MoCA (average ± SD) | 21.06 ± 4.65 |
| MMSE (average ± SD) | 26.67 ± 1.20 |
| Sex | |
| Male, % (n) | 57 (57) |
| Female, % (n) | 43 (43) |
| Diagnosis | |
| PD, % (n) | 65 (65) |
| Early onset PD, % (n) | 23 (23) |
| Youth PD, % (n) | 3 (3) |
| Family PD, % (n) | 6 (6) |
| Not defined, % (n) | 2 (2) |
| Presence of family history | 13 (13) |
| PPH | |
| None, % (n) | 67 (67) |
| T2D, % (n) | 11 (11) |
| AH, % (n) | 11 (11) |
| Other, % (n) | 18 (18) |
| Symptoms at the beginning of the disease | |
| Tremor, % (n) | 67 (67) |
| Rigidity, % (n) | 24 (24) |
| Gait disturbances, % (n) | 7 (7) |
| Strength disturbances, % (n) | 5 (5) |
| Bradykinesia, % (n) | 2 (2) |
| Side of onset of the disease | |
| Right, % (n) | 57 (57) |
| Left, % (n) | 40 (40) |
| Bilateral, % (n) | 2 (2) |
| Education higher than high school, % (n) | 35 (35) |
| Economically productive, % (n) | 40 (40) |
| Married, % (n) | 68 868) |
| History of psychiatric illness, % (n) | 30 (30) |
| Consumes caffeine, % (n) | 66(66) |
| Consumes tobacco, % (n) | 15 (15) |
| Severity of MDD by MADRS | |
| Mild, % (n) | 51 (51) |
| Moderate, % (n) | 44 (44) |
| Severe, % (n) | 5 (5) |
| Cases of moderate to severe depression, % (n) | 49 (49) |
| Variable | % (n) |
|---|---|
| Antidepressant management | |
| SSRIs | 69 (69) |
| Dual antidepressant | 20 (20) |
| Mirtazpine | 5 (5) |
| Tricyclic antidepressant | 13 (13) |
| Trazodone | 1 (1) |
| PD management | |
| Donepezil | 1 (1) |
| Pramipexole | 52 (52) |
| Galantamine | 1 (1) |
| Bromocriptine | 4 (4) |
| Trihexiphenidyl | 2 (2) |
| Leflunomide | 1(1) |
| Rotigotine | 5 (5) |
| Levodopa | 2 (2) |
| Levodopa/Carbidopa | 80 (80) |
| Levodopa/Benserazide | 5 (5) |
| Levodopa/Carbidopa/Entacapona | 13 (13) |
| Selegiline | 7 (7) |
| Rasagiline | 1 (1) |
| Amantadine | 22 (22) |
| Biperiden | 13 (13) |
| Propanolol | 3 (3) |
| Variable | Male (n = 57) | Female (n = 43) | p < 0.05 | Mild MDD (n = 51) | M/S MDD (n = 49) | p < 0.05 |
|---|---|---|---|---|---|---|
| Age (years, average ± SD) | 58.82 ± 11.42 | 58.05 ± 10.71 | 0.365 | 58.57 ± 10.75 | 57.92 ± 11.47 | 0.387 |
| Age of onset PD (years, average ± SD) | 50.65 ± 12.20 | 50.67 ± 11.68 | 0.496 | 50.96 ± 11.41 | 49.6 ± 12.47 | 0.291 |
| Years of evolution PD (average ± SD) | 8.18 ± 4.84 | 7.37 ± 6.00 | 0.230 | 7.62 ± 5.00 | 8.31 ± 5.84 | 0.268 |
| UPDRS III (average ± SD) | 29.80 ± 16.37 | 44.00 ± 16.03 | 0.188 | 28.35 ± 14.22 | 33.73 ± 18.1 | 0.134 |
| MADRS (average ± SD) | 18.41 ± 7.29 | 21.31 ± 8.10 | 0.028 | 13.43 ± 3.79 | 25.44 ± 5.66 | <0.001 |
| MOCA (average ± SD) | 21.52 ± 4.51 | 20.41 ± 4.91 | 0.122 | 21.4 ± 4.51 | 20.77 ± 4.94 | 0.258 |
| MMSE (average ± SD) | 26.22 ± 2.97 | 25.03 ± 3.58 | 0.043 | 25.93 ± 2.92 | 25.51 ± 3.63 | 0.281 |
| Sex | ||||||
| Male | - | - | NA | 62.75 (32) | 51.06 (25) | 0.085 |
| Female | - | - | NA | 37.25 (19) | 48.94 (24) | 0.085 |
| PPH | ||||||
| Presence of family history, % (n) | 12.73 (7) | 12.82 (6) | 0.403 | 10.64 (5) | 14.89 (7) | 0.281 |
| None, % (n) | 69.09 (39) | 64.10 (28) | 0.288 | 61.70 (31) | 72.34 (35) | 0.202 |
| T2D, % (n) | 14.55(8) | 5.13 (2) | 0.134 | 10.64 (5) | 10.64 (5) | 0.347 |
| AH, % (n) | 7.27 (4) | 15.38 (7) | 0.070 | 8.51 (4) | 12.77 (6) | 0.395 |
| Other, % (n) | 14.55(8) | 23.08 (10) | 0.178 | 23.40 (12) | 12.77 (6) | 0.125 |
| Symptoms at the onset of the disease | ||||||
| Tremor, % (n) | 36 (63.64) | 71.79 (31) | 0.154 | 74.47 (37) | 59.57 (29) | 0.072 |
| Rigidity, % (n) | 11 (20.00) | 30.77 (13) | 0.103 | 12.77 (6) | 36.17 (18) | 0.005 |
| Gait disturbances, % (n) | 7.27 (4) | 7.69 (3) | 0.497 | 12.77 (6) | 2.13 (1) | 0.023 |
| Strength disturbances, % (n) | 7.27 (4) | 2.56 (1) | 0.143 | 4.26 (2) | 6.38 (3) | 0.332 |
| Bradykinesia, % (n) | 1.82 (1) | 2.56 (1) | 0.420 | 2.13 (1) | 2.13 (1) | 0.494 |
| Side of onset of the disease | ||||||
| Right, % (n) | 54.55 (31) | 61.54 (26) | 0.292 | 63.83 (32) | 51.06 (25) | 0.087 |
| Left, % (n) | 41.82 (24) | 38.46 (17) | 0.421 | 31.91 (16) | 48.94 (24) | 0.037 |
| Bilateral, % (n) | 3.64 (2) | 0.00 (0) | 0.107 | 4.26 (2) | 0.00 (0) | 0.074 |
| Sociodemographic variables | ||||||
| Education higher than high school, % (n) | 43.64 (25) | 23.08 (10) | 0.043 | 29.79 (15) | 40.43 (20) | 0.158 |
| Economically productive, % (n) | 38.18 (22) | 43.59 (19) | 0.165 | 46.81 (23) | 34.04 (17) | 0.09 |
| Married, % (n) | 80.00 (46) | 51.28 (22) | 0.002 | 68.09 (34) | 68.09 (33) | 0.472 |
| History of psychiatric illness, % (n) | 23.64 (13) | 38.46 (17) | 0.086 | 23.40 (12) | 36.17 (18) | 0.1 |
| Consumes caffeine, % (n) | 69.09 (39) | 61.54 (26) | 0.250 | 65.96 (33) | 65.96 (32) | 0.471 |
| Consumes tobacco, % (n) | 23.64 (13) | 2.56 (1) | 0.003 | 21.28 (11) | 8.51 (4) | 0.073 |
| Severity of MDD by MADRS | ||||||
| Mild, % (n) | 56.14 (32) | 44.19 (19) | 0.194 | - | - | NA |
| Moderate, % (n) | 40.35 (23) | 48.84 (21) | 0.163 | - | - | NA |
| Severe, % (n) | 3.51 (2) | 6.98 (3) | 0.188 | - | - | NA |
| Drug variables | ||||||
| SSRI, % (n) | 70.18 (40) | 72.09 (31) | 0.417 | 74.51 (38) | 67.35 (33) | 0.215 |
| Dual, % (n) | 17.54 (10) | 20.93 (9) | 0.335 | 13.73 (7) | 24.49 (12) | 0.085 |
| Mirtazapine, % (n) | 8.77 (5) | 2.33 (1) | 0.090 | 9.80 (5) | 2.04 (1) | 0.051 |
| Tricyclic, % (n) | 10.53 (6) | 13.95 (6) | 0.301 | 11.76 (6) | 12.24 (6) | 0.471 |
| Trazodone, % (n) | 0.00 (0) | 2.33 (1) | 0.124 | 1.96 (1) | 0.00 (0) | 0.162 |
| Donepezil, % (n) | 0.00 (0) | 2.33 (1) | 0.124 | 1.96 (1) | 0.00 (0) | 0.162 |
| Pramipexole, % (n) | 47.37 (27) | 53.49 (23) | 0.272 | 45.10 (23) | 55.10 (27) | 0.159 |
| Galantamine, % (n) | 0.00 (0) | 2.33 (1) | 0.191 | 0.00 (0) | 2.04 (1) | 0.153 |
| Bromocriptine, % (n) | 3.51 (2) | 4.65 (2) | 0.386 | 5.88 (3) | 2.04 (1) | 0.164 |
| Trihexiphenidyl, % (n) | 1.75 (1) | 2.33 (1) | 0.580 | 0.00 (0) | 4.08 (2) | 0.073 |
| Leflunomide, % (n) | 0.00 (0) | 2.33 (1) | 0.124 | 1.96 (1) | 0.00 (0) | 1.000 |
| Rotigotine, % (n) | 7.02 (4) | 6.98 (3) | 0.503 | 5.88 (3) | 8.16 (4) | 0.328 |
| Levodopa, % (n) | 3.51 (2) | 0.00 (0) | 0.215 | 1.96 (1) | 2.04 (1) | 0.489 |
| Levodopa/Carbidopa, % (n) | 80.70 (46) | 79.07 (34) | 0.420 | 84.31 (43) | 75.51 (37) | 0.136 |
| Levodopa/Benserazide, % (n) | 5.26 (3) | 74.42 (32) | 0.445 | 3.92 (2) | 67.35 (33) | 0.307 |
| Levodopa/Carbidopa/Entacapona, % (n) | 12.28 (7) | 13.95 (6) | 0.403 | 13.73 (7) | 12.24 (6) | 0.413 |
| Selegiline, % (n) | 3.51 (2) | 13.95 (6) | 0.057 | 5.88 (3) | 10.20 (5) | 0.213 |
| Rasagiline, % (n) | 3.51 (2) | 0.00 (0) | 0.107 | 1.96 (1) | 2.04 (1) | 0.489 |
| Amantadine, % (n) | 19.30 (11) | 25.58 (11) | 0.226 | 23.53 (12) | 20.41 (10) | 0.353 |
| Biperiden, % (n) | 15.79 (9) | 9.30 (4) | 0.170 | 13.73 (7) | 12.24 (6) | 0.413 |
| Propanolol, % (n) | 3.51 (2) | 2.33 (1) | 0.366 | 1.96 (1) | 4.08 (2) | 0.267 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janssen-Aguilar, R.; Rojas, P.; Ruiz-Sánchez, E.; Rodriguez-Violante, M.; Alcántara-Flores, Y.M.; Crail-Meléndez, D.; Cervantes-Arriaga, A.; Sánchez-Escandón, Ó.; Ruiz-Chow, Á.A. Naturalistic Study of Depression Associated with Parkinson’s Disease in a National Public Neurological Referral Center in Mexico. Brain Sci. 2022, 12, 326. https://doi.org/10.3390/brainsci12030326
Janssen-Aguilar R, Rojas P, Ruiz-Sánchez E, Rodriguez-Violante M, Alcántara-Flores YM, Crail-Meléndez D, Cervantes-Arriaga A, Sánchez-Escandón Ó, Ruiz-Chow ÁA. Naturalistic Study of Depression Associated with Parkinson’s Disease in a National Public Neurological Referral Center in Mexico. Brain Sciences. 2022; 12(3):326. https://doi.org/10.3390/brainsci12030326
Chicago/Turabian StyleJanssen-Aguilar, Reinhard, Patricia Rojas, Elizabeth Ruiz-Sánchez, Mayela Rodriguez-Violante, Yessica M. Alcántara-Flores, Daniel Crail-Meléndez, Amin Cervantes-Arriaga, Óscar Sánchez-Escandón, and Ángel A. Ruiz-Chow. 2022. "Naturalistic Study of Depression Associated with Parkinson’s Disease in a National Public Neurological Referral Center in Mexico" Brain Sciences 12, no. 3: 326. https://doi.org/10.3390/brainsci12030326