Efficacy of Facial Exercises in Facial Expression Categorization in Schizophrenia
Abstract
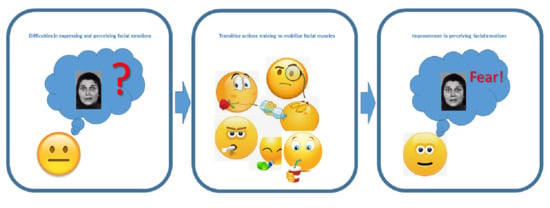
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Facial Physical Training
2.3. Facial Expression Categorization Test
2.3.1. Procedure
2.3.2. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Prim. 2015, 1, 15067. [Google Scholar] [CrossRef]
- Galderisi, S.; Mucci, A.; Buchanan, R.W.; Arango, C. Negative symptoms of schizophrenia: New developments and unanswered research questions. Lancet Psychiatr. 2018, 5, 664–677. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Buchanan, R.W.; Ross, D.E.; Carpenter, J. A separate disease within the syndrome of schizophrenia. Arch. Gen. Psychiatr. 2001, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Milev, P.; Ho, B.C.; Arndt, S.; Andreasen, N.C. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: A longitudinal first-episode study with 7-year follow-up. Am. J. Psychiatr. 2005, 162, 495–506. [Google Scholar] [CrossRef]
- An Der Heiden, W.; Häfner, H. The epidemiology of onset and course of schizophrenia. Eur. Arch. Psychiatr. Clin. Neurosci. 2000, 250, 292–303. [Google Scholar] [CrossRef]
- Carbon, M.; Correll, C.U. Thinking and acting beyond the positive: The role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 2014, 19, 38–52. [Google Scholar] [CrossRef]
- Künecke, J.; Hildebrandt, A.; Recio, G.; Sommer, W.; Wilhelm, O. Facial EMG responses to emotional expressions are related to emotion perception ability. PLoS ONE 2014, 9, e84053. [Google Scholar] [CrossRef]
- Ipser, A.; Cook, R. Inducing a concurrent motor load reduces categorization precision for facial expressions. J. Exp. Psychol. Hum. Percept. Perform. 2016, 42, 706–718. [Google Scholar] [CrossRef]
- Adolphs, R. Recognizing emotion from facial expressions: Psychological and neurological mechanisms. Behav. Cogn. Neurosci. Rev. 2002, 1, 21–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, J.; Jackson, H.J.; Pattison, P.E. Emotion recognition via facial expression and affective prosody in schizophrenia: A methodological review. Clin. Psychol. Rev. 2002, 22, 789–832. [Google Scholar] [CrossRef]
- Kohler, C.G.; Walker, J.B.; Martin, E.A.; Healey, K.M.; Moberg, P.J. Facial emotion perception in schizophrenia: A meta-analytic review. Schizophr. Bull. 2010, 36, 1009–1019. [Google Scholar] [CrossRef]
- Kucharska-Pietura, K.; David, A.S.; Masiak, M.; Phillips, M.L. Perception of facial and vocal affect by people with schizophrenia in early and late stages of illness. Br. J. Psychiatr. 2005, 187, 523–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archer, J.; Hay, D.C.; Young, A.W. Movement, face processing and schizophrenia: Evidence of a differential deficit in expression analysis. Br. J. Clin. Psychol. 1994, 33, 517–528. [Google Scholar] [CrossRef]
- Bellack, A.S.; Blanchard, J.J.; Mueser, K.T. Cue Availability and Affect Perception in Schizophrenia. Schizophr. Bull. 1996, 22, 535–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaebel, W.; Wölwer, W. Facial expression and emotional face recognition in schizophrenia and depression. Eur. Arch. Psychiatr. Clin. Neurosci. 1992, 242, 46–52. [Google Scholar] [CrossRef]
- Schneider, F.; Gur, R.C.; Gur, R.E.; Shtasel, D.L. Emotional processing in schizophrenia: Neurobehavioral probes in relation to psychopathology. Schizophr. Res. 1995, 17, 67–75. [Google Scholar] [CrossRef]
- Edwards, J.; Pattison, P.E.; Jackson, H.J.; Wales, R.J. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr. Res. 2001, 48, 235–253. [Google Scholar] [CrossRef]
- Amminger, G.P.; Schäfer, M.R.; Papageorgiou, K.; Klier, C.M.; Schlögelhofer, M.; Mossaheb, N.; Werneck-Rohrer, S.; Nelson, B.; McGorry, P.D. Emotion recognition in individuals at clinical high-risk for schizophrenia. Schizophr. Bull. 2012, 38, 1030–1039. [Google Scholar] [CrossRef]
- Comparelli, A.; Corigliano, V.; De Carolis, A.; Mancinelli, I.; Trovini, G.; Ottavi, G.; Dehning, J.; Tatarelli, R.; Brugnoli, R.; Girardi, P. Emotion recognition impairment is present early and is stable throughout the course of schizophrenia. Schizophr. Res. 2013, 143, 65–69. [Google Scholar] [CrossRef]
- Mele, S.; Bivi, R.; Borra, L.; Callegari, V.; Caracciolo, S.; Tugnoli, S.; Craighero, L. Efficacy of theatre activities in facial expression categorization in schizophrenia. Arts Psychother. 2019, 63, 141–150. [Google Scholar] [CrossRef]
- Mele, S.; Ghirardi, V.; Craighero, L. Facial expressions as a model to test the role of the sensorimotor system in the visual perception of the actions. Exp. Brain Res. 2017, 235, 3771–3783. [Google Scholar] [CrossRef]
- Niedenthal, P.M. Embodying emotion. Science 2007, 316, 1002–1005. [Google Scholar] [CrossRef] [Green Version]
- Niedenthal, P.M.; Mermillod, M.; Maringer, M.; Hess, U. The Simulation of Smiles (SIMS) model: Embodied simulation and the meaning of facial expression. Behav. Brain Sci. 2010, 33, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Price, T.F.; Harmon-Jones, E. Embodied emotion: The influence of manipulated facial and bodily states on emotive responses. WIREs Cogn. Sci. 2015, 6, 461–473. [Google Scholar] [CrossRef]
- Prenger, M.T.M.; Macdonald, P.A. Problems with Facial Mimicry Might Contribute to Emotion Recognition Impairment in Parkinson’s Disease. Parkinsons Dis. 2018, 2018, 5741941. [Google Scholar] [CrossRef] [Green Version]
- De Stefani, E.; Nicolini, Y.; Belluardo, M.; Ferrari, P.F. Congenital facial palsy and emotion processing: The case of Moebius syndrome. Genes Brain Behav. 2019, 18, e12548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekman, P.; Friesen, W.V. Pictures of Facial Affect; Consulting Psychologists Press: Palo Alto, CA, USA, 1976. [Google Scholar]
- Ekman, P.; Friesen, W.V.; Hager, J.C. The Facial Action Coding System; Consulting Psychologists Press: Palo Alto, CA, USA, 1975; Volume 50, ISBN 0931835011. [Google Scholar]
- Rosenbaum, S.; Tiedemann, A.; Sherrington, C.; Curtis, J.; Ward, P.B. Physical activity interventions for people with mental illness: A systematic review and meta-analysis. J. Clin. Psychiatr. 2014, 75, 964–974. [Google Scholar] [CrossRef]
- Vancampfort, D.; Correll, C.U.; Scheewe, T.W.; Probst, M.; De Herdt, A.; Knapen, J.; De Hert, M. Progressive muscle relaxation in persons with schizophrenia: A systematic review of randomized controlled trials. Clin. Rehabil. 2013, 27, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.; Mitchell, A.J.; De Hert, M.; Correll, C.U.; Soundy, A.; Stroobants, M.; Vancampfort, D. The prevalence and moderators of clinical pain in people with schizophrenia: A systematic review and large scale meta-analysis. Schizophr. Res. 2014, 160, 1–8. [Google Scholar] [CrossRef]
- Vancampfort, D.; Probst, M.; Skjaerven, L.H.; Catalán-Matamoros, D.; Lundvik-Gyllensten, A.; Gómez-Conesa, A.; Ijntema, R.; de Hert, M. Systematic review of the benefits of physical therapy within a multidisciplinary care approach for people with schizophrenia. Phys. Ther. 2012, 92, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Vera-Garcia, E.; Mayoral-Cleries, F.; Vancampfort, D.; Stubbs, B.; Cuesta-Vargas, A.I. A systematic review of the benefits of physical therapy within a multidisciplinary care approach for people with schizophrenia: An update. Psychiatr. Res. 2015, 229, 828–839. [Google Scholar] [CrossRef]
- Brach, J.S.; VanSwearingen, J.M.; Lenert, J.; Johnson, P.C. Facial neuromuscular retraining for oral synkinesis. Plast. Reconstr. Surg. 1997, 99, 1922–1933. [Google Scholar] [CrossRef]
- VanSwearingen, J.M.; Brach, J.S. Changes in facial movement and synkinesis with facial neuromuscular reeducation. Plast. Reconstr. Surg. 2003, 111, 2370–2375. [Google Scholar] [CrossRef]
- Manikandan, N. Effect of facial neuromuscular re-education on facial symmetry in patients with Bell’s palsy: A randomized controlled trial. Clin. Rehabil. 2007, 21, 338–343. [Google Scholar] [CrossRef]
- VanSwearingen, J. Facial rehabilitation: A neuromuscular reeducation, patient-centered approach. Facial Plast. Surg. 2008, 24, 250–259. [Google Scholar] [CrossRef]
- Baumel, J.J. Trigeminal-Facial Nerve Communications: Their Function in Facial Muscle Innervation and Reinnervation. Arch. Otolaryngol. 1974, 99, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Burgess, P.R.; Wei, J.Y.; Clark, F.J.; Simon, J. Signaling of kinesthetic information by peripheral sensory receptors. Annu. Rev. Neurosci. 1982, 5, 171–187. [Google Scholar] [CrossRef]
- Ekman, P.; Friesen, W.V.; Hager, J.C. Emotional Facial Action Coding System. Manual and Investigator’s Guide; Research Nexus: Salt Lake City, UT, USA, 2002. [Google Scholar]
- Buccino, G. Action observation treatment: A novel tool in neurorehabilitation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130185. [Google Scholar] [CrossRef] [PubMed]
- Pollak, S.D.; Kistler, D.J. Early experience is associated with the development of categorical representations for facial expressions of emotion. Proc. Natl. Acad. Sci. USA 2002, 99, 9072–9076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decety, J.; Chaminade, T. The Neurophysiology of Imitation and Intersubjectivity. In Perspectives on Imitation; Hurley, S., Chater, N., Eds.; The MIT Press: Cambridge, MA, USA, 2005; pp. 119–140. [Google Scholar]
- Gallese, V. The roots of empathy: The shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology 2003, 36, 171–180. [Google Scholar] [CrossRef]
- Gallese, V. Embodied Simulation: From Mirror Neuron Systems to Interpersonal Relations. In Empathy and Fairness; Wiley: New York, NY, USA, 2008; pp. 3–12. ISBN 9780470030585. [Google Scholar]
- Keysers, C.; Gazzola, V. Integrating simulation and theory of mind: From self to social cognition. Trends Cogn. Sci. 2007, 11, 194–196. [Google Scholar] [CrossRef]
- Dimberg, U. Facial Reactions to Facial Expressions. Psychophysiology 1982, 19, 643–647. [Google Scholar] [CrossRef]
- Korb, S.; Grandjean, D.; Scherer, K.R. Timing and voluntary suppression of facial mimicry to smiling faces in a Go/NoGo task-An EMG study. Biol. Psychol. 2010, 85, 347–349. [Google Scholar] [CrossRef]
- Dimberg, U.; Thunberg, M.; Grunedal, S. Facial reactions to emotional stimuli: Automatically controlled emotional responses. Cogn. Emot. 2002, 16, 449–471. [Google Scholar] [CrossRef]
- Bornemann, B.; Winkielman, P.; van der Meer, E. Can you feel what you do not see? Using internal feedback to detect briefly presented emotional stimuli. Int. J. Psychophysiol. 2012, 85, 116–124. [Google Scholar] [CrossRef]
- Dimberg, U.; Thunberg, M.; Elmehed, K. Unconscious facial reactions to emotional facial expressions. Psychol. Sci. 2000, 11, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Bulnes, L.C.; Mariën, P.; Vandekerckhove, M.; Cleeremans, A. The effects of Botulinum toxin on the detection of gradual changes in facial emotion. Sci. Rep. 2019, 9, 11734. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.D.; Winkielman, P.; Coulson, S. Sensorimotor simulation and emotion processing: Impairing facial action increases semantic retrieval demands. Cogn. Affect. Behav. Neurosci. 2017, 17, 652–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedenthal, P.M.; Brauer, M.; Halberstadt, J.B.; Innes-Ker, Å.H. When did her smile drop? Facial mimicry and the influences of emotional state on the detection of change in emotional expression. Cogn. Emot. 2001, 15, 853–864. [Google Scholar] [CrossRef]
- Oberman, L.M.; Winkielman, P.; Ramachandran, V.S. Face to face: Blocking facial mimicry can selectively impair recognition of emotional expressions. Soc. Neurosci. 2007, 2, 167–178. [Google Scholar] [CrossRef]
- Neal, D.T.; Chartrand, T.L. Embodied emotion perception: Amplifying and dampening facial feedback modulates emotion perception accuracy. Soc. Psychol. Personal. Sci. 2011, 2, 673–678. [Google Scholar] [CrossRef]
- Stel, M.; Van Knippenberg, A. The role of facial mimicry in the recognition of affect. Psychol. Sci. 2008, 19, 984–985. [Google Scholar] [CrossRef] [PubMed]
- Kring, A.M.; Moran, E.K. Emotional response deficits in schizophrenia: Insights from affective science. Schizophr. Bull. 2008, 34, 819–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenbaum, H.; Oltmanns, T.F. Emotional Experience and Expression in Schizophrenia and Depression. J. Abnorm. Psychol. 1992, 101, 37–44. [Google Scholar] [CrossRef]
- Salem, J.E.; Kring, A.M. Flat affect and social skills in schizophrenia: Evidence for their independence. Psychiatr. Res. 1999, 87, 159–167. [Google Scholar] [CrossRef]
- Earnst, K.S.; Kring, A.M. Emotional responding in deficit and non-deficit schizophrenia. Psychiatr. Res. 1999, 88, 191–207. [Google Scholar] [CrossRef]
- Henry, J.D.; Green, M.J.; de Lucia, A.; Restuccia, C.; McDonald, S.; O’Donnell, M. Emotion dysregulation in schizophrenia: Reduced amplification of emotional expression is associated with emotional blunting. Schizophr. Res. 2007, 95, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kring, A.M.; Earnst, K.S. Stability of emotional responding in schizophrenia. Behav. Ther. 1999, 30, 373–388. [Google Scholar] [CrossRef]
- Kring, A.M.; Kerr, S.L.; Smith, D.A.; Neale, J.M. Flat Affect in Schizophrenia Does Not Reflect Diminished Subjective Experience of Emotion. J. Abnorm. Psychol. 1993, 102, 507–517. [Google Scholar] [CrossRef]
- Kring, A.M.; Neale, J.M. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? J. Abnorm. Psychol. 1996, 105, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.M.; Schneider, F.; Heimann, H.; Birbaumer, N. Reduced emotional response of schizophrenic patients in remission during social interaction. Schizophr. Res. 1995, 17, 249–255. [Google Scholar] [CrossRef]
- Blanchard, J.J.; Sayers, S.L.; Collins, L.M.; Bellack, A.S. Affectivity in the problem-solving interactions of schizophrenia patients and their family members. Schizophr. Res. 2004, 69, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Borod, J.C.; Alpert, M.; Brozgold, A.; Martin, C.; Welkowitz, J.; Diller, L.; Peselow, E.; Angrist, B.; Lieberman, A. A preliminary comparison of flat affect schizophrenics and brain-damaged patients on meausres of affective processing. J. Commun. Disord. 1989, 22, 93–104. [Google Scholar] [CrossRef]
- Gaebel, W.; Wölwer, W. Facial expressivity in the course of schizophrenia and depression. Eur. Arch. Psychiatr. Clin. Neurosci. 2004, 254, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Krause, R.; Steimer, E.; Sänger-Alt, C.; Wagner, G. Facial Expression of Schizophrenic Patients and Their Interaction Partners. Psychiatry 1989, 52, 1–12. [Google Scholar] [CrossRef]
- Kring, A.M.; Alpert, M.; Neale, J.M.; Harvey, P.D. A multimethod, multichannel assessment of affective flattening in schizophrenia. Psychiatr. Res. 1994, 54, 211–222. [Google Scholar] [CrossRef]
- Martin, C.C.; Borod, J.C.; Alpert, M.; Brozgold, A.; Welkowitz, J. Spontaneous expression of facial emotion in schizophrenic and right-brain-damaged patients. J. Commun. Disord. 1990, 23, 287–301. [Google Scholar] [CrossRef]
- Trémeau, F.; Malaspina, D.; Duval, F.; Corrêa, H.; Hager-Budny, M.; Coin-Bariou, L.; Macher, J.P.; Gorman, J.M. Facial expressiveness in patients with schizophrenia compared to depressed patients and nonpatient comparison subjects. Am. J. Psychiatr. 2005, 162, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Aghevli, M.A.; Blanchard, J.J.; Horan, W.P. The expression and experience of emotion in schizophrenia: A study of social interactions. Psychiatr. Res. 2003, 119, 261–270. [Google Scholar] [CrossRef]
- Horan, W.P.; Kern, R.S.; Green, M.F.; Penn, D.L. Social cognition training for individuals with schizophrenia: Emerging evidence. Am. J. Psychiatr. Rehabil. 2008, 11, 205–252. [Google Scholar] [CrossRef]
- Jin, H.C.; Jin, H.K.; Lee, J.; Green, M.F. Social cognition training for individuals with schizophrenia: A review of targeted interventions. Clin. Psychopharmacol. Neurosci. 2009, 7, 29–38. [Google Scholar]
- Wölwer, W.; Combs, D.R.; Frommann, N.; Penn, D.L. Treatment approaches with a special focus on social cognition: Overview and empirical results. In Neurocognition and Social Cognition in Schizophrenia Patients: Basic Concepts and Treatment; Karger Medical and Scientific Publishers: Basel, Switzerland, 2010; Volume 177, pp. 61–78. ISBN 9783805593397. [Google Scholar]
- Wölwer, W.; Frommann, N. Social-cognitive remediation in schizophrenia: Generalization of effects of the training of affect recognition (TAR). Schizophr. Bull. 2011, 37, S63–S70. [Google Scholar] [CrossRef] [Green Version]
- Wölwer, W.; Frommann, N.; Halfmann, S.; Piaszek, A.; Streit, M.; Gaebel, W. Remediation of impairments in facial affect recognition in schizophrenia: Efficacy and specificity of a new training program. Schizophr. Res. 2005, 80, 295–303. [Google Scholar] [CrossRef]
- Frommann, N.; Streit, M.; Wölwer, W. Remediation of facial affect recognition impairments in patients with schizophrenia: A new training program. Psychiatr. Res. 2003, 117, 281–284. [Google Scholar] [CrossRef]
- Gorczynski, P.; Faulkner, G. Exercise therapy for schizophrenia. Schizophr. Bull. 2010, 36. [Google Scholar] [CrossRef]
- Firth, J.; Cotter, J.; Elliott, R.; French, P.; Yung, A.R. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol. Med. 2015, 45, 1343–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Experimental Group | Control Group | ||||||
|---|---|---|---|---|---|---|---|
| Age | Gender | ICD-10 Code | Main Symptoms | Age | Gender | ICD-10 Code | Main Symptoms |
| 57 | M | F 20.5 | Affective flattening; poverty of speech; reduced social functioning | 33 | M | F 20.0 | Chronic persecutory delusional disorder; auditory hallucinations |
| 62 | M | F 25.2 | Persecutory delusional ideas; emotional instability; relational isolation | 50 | M | F 20.0 | Chronic persecutory delusional disorder |
| 45 | F | F 25.2 | Affective instability; delusional persecutory cues; incongruity of thought | 40 | F | F 20.1 | Alterations of affectivity; fluctuating hallucinations; disorganized behavior and thinking |
| 57 | F | F 20.0 | Persecutory delusional ideation; auditory hallucinations | 56 | M | F 20.0 | Chronic persecutory and erotomanic delusional disorder; auditory hallucinations |
| 41 | F | F 25.2 | Affective instability; delusional persecutory cues; incongruity of thought | 34 | F | F 20.0 | Chronic persecutory delusional disorder |
| 35 | F | F 20.1 | Alterations of affectivity; fluctuating and fragmentary delusions; unpredictable behavior | 54 | F | F 20.0 | Mystical Chronic Delusional Disorder |
| 53 | M | F 20.5 | Affective flattening; poverty of speech; reduced social functioning | 53 | M | F 20.0 | Chronic persecutory delusional disorder |
| 63 | F | F 20.1 | Alterations of affectivity; fluctuating delusions and hallucinations; mannerisms; fatuous and inappropriate mood; disorganized thinking; incoherent speech | 42 | M | F 25.2 | Persecutory delusional ideas; mind reading; emotional instability |
| 55 | F | F 20.1 | Affective flattening; loss of initiative; social isolation | 66 | M | F 20.0 | Chronic persecutory delusional disorder; apathy; abulia; relational isolation |
| 52 | M | F 20.5 | Affective flattening; poverty of speech; reduced social functioning | 56 | M | F 20.0 | Chronic persecutory delusional disorder; apathy; abulia; relational isolation |
| 58 | F | F 20.0 | Chronic persecutory delusional disorder | 49 | M | F 20.5 | Psychomotor slowdown; psychoaffective flattening; passivity and lack of initiative; poverty of speech |
| 62 | F | F 20.1 | Affective flattening; loss of initiative; social isolation | 45 | M | F 20.5 | Affective flattening; poverty of speech; reduced social functioning |
| Emotion | Action Units (AUs) |
|---|---|
| Anger | 4 + 5 + 7 + 23 |
| Fear | 1 + 2 + 4 + 5 + 7 + 20 + 26 |
| Happiness | 6 + 12 |
| Sadness | 1 + 4 + 15 |
| AU Number | Action Descriptor | Muscular Basis |
|---|---|---|
| 1 | Inner brow raiser | Frontalis (Pars Medialis) |
| 2 | Outer brow raiser | Frontalis (Pars Lateralis) |
| 4 | Brow lowerer | Depressor Glabellae Depressor Supercilii Corrugator Supercilii |
| 5 | Upper lid raiser | Levator Palpebrae Superioris Superior Tarsal Muscle |
| 6 | Cheek raiser | Orbicularis Oculi (Pars Orbitalis) |
| 7 | Lid tightener | Orbicularis Oculi (Pars Palpebralis) |
| 12 | Lip corner puller | Zygomaticus Major |
| 15 | Lip corner depressor | Depressor Anguli Oris |
| 20 | Lip stretcher | Risorius, Platysma |
| 23 | Lip tightener | Orbicularis Oris |
| 26 | Jaw drop | Masseter |
| Facial District | Exercise |
|---|---|
| Mobilization of the lips | hold a stick horizontally between the teeth without touching it with the lips hold a stick horizontally between the lips without touching it with the teeth hold a stick vertically between the lips without touching it with the teeth hold objects of varying heaviness between the upper lip and nose hold round objects of various sizes and textures between the lips push a small ball towards a target by blowing through a straw make bubbles in different amounts of water by blowing through a straw inflate balloons with different resistance make soap bubbles |
| Mobilization of the muscles around the eyes | move the glasses placed on the nose upwards using the cheek muscles hold round objects of various sizes by tightly contracting the muscles around both eyes hold round objects of various sizes by tightly contracting the muscles around one eye, and look with the other eye remove a little piece of paper placed between the eyebrows by frowning |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pancotti, F.; Mele, S.; Callegari, V.; Bivi, R.; Saracino, F.; Craighero, L. Efficacy of Facial Exercises in Facial Expression Categorization in Schizophrenia. Brain Sci. 2021, 11, 825. https://doi.org/10.3390/brainsci11070825
Pancotti F, Mele S, Callegari V, Bivi R, Saracino F, Craighero L. Efficacy of Facial Exercises in Facial Expression Categorization in Schizophrenia. Brain Sciences. 2021; 11(7):825. https://doi.org/10.3390/brainsci11070825
Chicago/Turabian StylePancotti, Francesco, Sonia Mele, Vincenzo Callegari, Raffaella Bivi, Francesca Saracino, and Laila Craighero. 2021. "Efficacy of Facial Exercises in Facial Expression Categorization in Schizophrenia" Brain Sciences 11, no. 7: 825. https://doi.org/10.3390/brainsci11070825