Oxidative Stress and Neurodevelopmental Outcomes in Rat Offspring with Intrauterine Growth Restriction Induced by Reduced Uterine Perfusion
Abstract
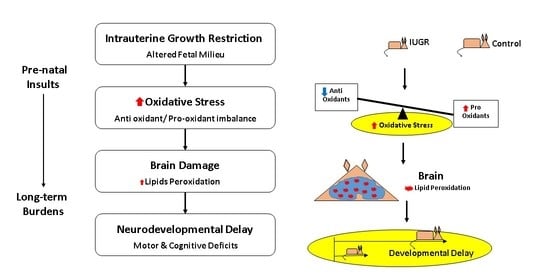
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Reduced Uterine Perfusion (RUP) Procedure in the Pregnant Rat
2.3. Behavioral Testing
2.3.1. Grip Strength Assessment
2.3.2. Open Field Test
2.3.3. Novel Object Recognition
2.4. Determination of Oxidative Stress
2.4.1. Determination of Plasma Oxidative Markers
2.4.2. Measurement of Brain Lipid Peroxidation
2.5. Statistics
3. Results
3.1. Effects of Reduced Uterine Perfusion on Offspring’s Body Weight
3.2. Behavioral Test in Offspring from Dams Exposed to RUP
3.2.1. Grip Strength Assessment
3.2.2. Locomotor Activity
3.2.3. Novel Object Recognition
3.3. Molecular Measurements of Oxidative Stress
3.3.1. Pro-Oxidation Markers
3.3.2. Anti-Oxidative Stress Markers
3.3.3. Lipid Peroxidation
3.4. Correlation between Molecular Markers and Behavioral Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberry, M.; Soothill, P. Management of fetal growth restriction. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F62–F67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, D.M.; Marlow, N. Neurocognitive outcome following fetal growth restriction. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F322–F325. [Google Scholar] [CrossRef] [PubMed]
- Levine, T.A.; Grunau, R.E.; McAuliffe, F.M.; Pinnamaneni, R.; Foran, A.; Alderdice, F.A. Early childhood neurodevelopment after intrauterine growth restriction: A systematic review. Pediatrics 2015, 135, 126–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, U.; Bhalerao, S. Placental insufficiency and fetal growth restriction. J. Obs. Gynaecol. India 2011, 61, 505–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitner, Y.; Fattal-Valevski, A.; Geva, R.; Bassan, H.; Posner, E.; Kutai, M.; Many, A.; Jaffa, A.J.; Harel, S. Six-year follow-up of children with intrauterine growth retardation: Long-term, prospective study. J. Child. Neurol 2000, 15, 781–786. [Google Scholar] [CrossRef]
- Leitner, Y.; Fattal-Valevski, A.; Geva, R.; Eshel, R.; Toledano-Alhadef, H.; Rotstein, M.; Bassan, H.; Radianu, B.; Bitchonsky, O.; Jaffa, A.J.; et al. Neurodevelopmental outcome of children with intrauterine growth retardation: A longitudinal, 10-year prospective study. J. Child. Neurol. 2007, 22, 580–587. [Google Scholar] [CrossRef]
- Beltrand, J.; Nicolescu, R.; Kaguelidou, F.; Verkauskiene, R.; Sibony, O.; Chevenne, D.; Claris, O.; Levy-Marchal, C. Catch-up growth following fetal growth restriction promotes rapid restoration of fat mass but without metabolic consequences at one year of age. PLoS ONE 2009, 4, e5343. [Google Scholar] [CrossRef]
- Bellido-Gonzalez, M.; Diaz-Lopez, M.A.; Lopez-Criado, S.; Maldonado-Lozano, J. Cognitive Functioning and academic achievement in children aged 6-8 years, born at term after intrauterine growth restriction and fetal cerebral redistribution. J. Pediatr. Psychol. 2017, 42, 345–354. [Google Scholar] [CrossRef]
- Fushima, T.; Sekimoto, A.; Minato, T.; Ito, T.; Oe, Y.; Kisu, K.; Sato, E.; Funamoto, K.; Hayase, T.; Kimura, Y.; et al. Reduced uterine perfusion pressure (RUPP) model of preeclampsia in mice. PLoS ONE 2016, 11, e0155426. [Google Scholar] [CrossRef] [Green Version]
- Granger, J.P.; LaMarca, B.B.; Cockrell, K.; Sedeek, M.; Balzi, C.; Chandler, D.; Bennett, W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol. Med. 2006, 122, 383–392. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Bland, J.M.; Robertson, W.B.; Brosens, I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 1983, 4, 397–413. [Google Scholar] [CrossRef]
- Pardi, G.; Marconi, A.M.; Cetin, I. Placental-fetal interrelationship in IUGR fetuses—A review. Placenta 2002, 23 (Suppl. A), S136–S141. [Google Scholar] [CrossRef] [PubMed]
- Regnault, T.R.; Galan, H.L.; Parker, T.A.; Anthony, R.V. Placental development in normal and compromised pregnancies—A review. Placenta 2002, 23 (Suppl. A), S119–S129. [Google Scholar] [CrossRef]
- Stott, D.; Papastefanou, I.; Paraschiv, D.; Clark, K.; Kametas, N.A. Longitudinal maternal hemodynamics in pregnancies affected by fetal growth restriction. Ultrasound Obs. Gynecol. 2017, 49, 761–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regal, J.F.; Lillegard, K.E.; Bauer, A.J.; Elmquist, B.J.; Loeks-Johnson, A.C.; Gilbert, J.S. Neutrophil depletion attenuates placental ischemia-induced hypertension in the rat. PLoS ONE 2015, 10, e0132063. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Yung, H.W.; Cindrova-Davies, T.; Charnock-Jones, D.S. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta 2009, 30 (Suppl. A), S43–S48. [Google Scholar] [CrossRef] [Green Version]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Ferreira, D.J.S.; da Silva Pedroza, A.A.; Braz, G.R.F.; da Silva-Filho, R.C.; Lima, T.A.; Fernandes, M.P.; Doi, S.Q.; Lagranha, C.J. Mitochondrial bioenergetics and oxidative status disruption in brainstem of weaned rats: Immediate response to maternal protein restriction. Brain Res. 2016, 1642, 553–561. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Soleimani, E.; Goudarzi, I.; Abrari, K.; Lashkarbolouki, T. The combined effects of developmental lead and ethanol exposure on hippocampus dependent spatial learning and memory in rats: Role of oxidative stress. Food Chem. Toxicol. 2016, 96, 263–272. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.; Salem, N.A.; El-Shamarka, M.E.; Hussein, J.S.; Ahmed, N.A.; El-Nagar, M.E. Studies on the effects of aspartame on memory and oxidative stress in brain of mice. Eur. Rev. Med. Pharm. Sci. 2012, 16, 2092–2101. [Google Scholar]
- Xiaoli, F.; Junrong, W.; Xuan, L.; Yanli, Z.; Limin, W.; Jia, L.; Longquan, S. Prenatal exposure to nanosized zinc oxide in rats: Neurotoxicity and postnatal impaired learning and memory ability. Nanomed. (Lond.) 2017, 12, 777–795. [Google Scholar] [CrossRef] [PubMed]
- Merzoug, S.; Toumi, M.L.; Boukhris, N.; Baudin, B.; Tahraoui, A. Adriamycin-related anxiety-like behavior, brain oxidative stress and myelotoxicity in male Wistar rats. Pharm. Biochem. Behav. 2011, 99, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. The migraine attack as a homeostatic, neuroprotective response to brain oxidative stress: Preliminary evidence for a theory. Headache 2018, 58, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Stigger, F.; Lovatel, G.; Marques, M.; Bertoldi, K.; Moyses, F.; Elsner, V.; Siqueira, I.R.; Achaval, M.; Marcuzzo, S. Inflammatory response and oxidative stress in developing rat brain and its consequences on motor behavior following maternal administration of LPS and perinatal anoxia. Int. J. Dev. Neurosci. 2013, 31, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.P.; Gariepy Hde, B.; Ongali, B.; Couture, R. Brain kinin B1 receptor is upregulated by the oxidative stress and its activation leads to stereotypic nociceptive behavior in insulin-resistant rats. Peptides 2015, 69, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Reus, G.Z.; Becker, I.R.T.; Scaini, G.; Petronilho, F.; Oses, J.P.; Kaddurah-Daouk, R.; Ceretta, L.B.; Zugno, A.I.; Dal-Pizzol, F.; Quevedo, J.; et al. The inhibition of the kynurenine pathway prevents behavioral disturbances and oxidative stress in the brain of adult rats subjected to an animal model of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 55–63. [Google Scholar] [CrossRef]
- Coles, L.D.; Tuite, P.J.; Oz, G.; Mishra, U.R.; Kartha, R.V.; Sullivan, K.M.; Cloyd, J.C.; Terpstra, M. Repeated-dose oral n-acetylcysteine in Parkinson’s disease: Pharmacokinetics and effect on brain glutathione and oxidative stress. J. Clin. Pharm. 2018, 58, 158–167. [Google Scholar] [CrossRef]
- Ojeda, N.B.; Hennington, B.S.; Williamson, D.T.; Hill, M.L.; Betson, N.E.; Sartori-Valinotti, J.C.; Reckelhoff, J.F.; Royals, T.P.; Alexander, B.T. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension 2012, 60, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Ojeda, N.; Hall, S.; Lasley, C.J.; Rudsenske, B.; Dixit, M.; Arany, I. Prenatal nicotine exposure augments renal oxidative stress in embryos of pregnant rats with reduced uterine perfusion pressure. Vivo 2016, 30, 219–224. [Google Scholar]
- Campbell, L.R.; Pang, Y.; Ojeda, N.B.; Zheng, B.; Rhodes, P.G.; Alexander, B.T. Intracerebral lipopolysaccharide induces neuroinflammatory change and augmented brain injury in growth-restricted neonatal rats. Pediatr. Res. 2012, 71, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Eder, D.J.; McDonald, M.T. A role for brain angiotensin II in experimental pregnancy-induced hypertension in laboratory rats. Clin. Exp. Hypertens. Part B Hypertens. Pregnancy 1987, 6, 431–451. [Google Scholar] [CrossRef]
- Altman, J.; Sudarshan, K.; Das, G.D.; McCormick, N.; Barnes, D. The influence of nutrition on neural and behavioral development. 3. Development of some motor, particularly locomotor patterns during infancy. Dev. Psychobiol. 1971, 4, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Porcel, F.; Green, D.; Khatri, N.; Harris, S.S.; May, W.L.; Lin, R.C.; Paul, I.A. Neonatal exposure of rats to antidepressants affects behavioral reactions to novelty and social interactions in a manner analogous to autistic spectrum disorders. Anat. Rec. (Hoboken) 2011, 294, 1726–1735. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.T.; Lee, Y.J.; Lee, J.W.; Lu, S.; Tucci, M.A.; Dai, X.; Ojeda, N.B.; Lee, H.J.; Fan, L.W.; Tien, L.T. Interleukin-1 receptor antagonist ameliorates the pain hypersensitivity, spinal inflammation and oxidative stress induced by systemic lipopolysaccharide in neonatal rats. Neurochem. Int. 2020, 135, 104686. [Google Scholar] [CrossRef]
- Salminen, L.E.; Paul, R.H. Oxidative stress and genetic markers of suboptimal antioxidant defense in the aging brain: A theoretical review. Rev. Neurosci. 2014, 25, 805–819. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef]
- Coats, L.E.; Davis, G.K.; Newsome, A.D.; Ojeda, N.B.; Alexander, B.T. Low birth weight, blood pressure and renal susceptibility. Curr. Hypertens. Rep. 2019, 21, 62. [Google Scholar] [CrossRef]
- Peleg, D.; Kennedy, C.M.; Hunter, S.K. Intrauterine growth restriction: Identification and management. Am. Fam. Physician 1998, 58, 453–467. [Google Scholar]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharm. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Altafaj, X.; Dierssen, M.; Baamonde, C.; Marti, E.; Visa, J.; Guimera, J.; Oset, M.; Gonzalez, J.R.; Florez, J.; Fillat, C.; et al. Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down’s syndrome. Hum. Mol. Genet. 2001, 10, 1915–1923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 2013, 6, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, P.; Dupuis, R.; Costentin, J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994, 61, 59–64. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.Y.; MacQuarrie, R.A. Deacylation-reacylation of arachidonoyl groups in cerebral phospholipidsa. Ann. N. Y. Acad. Sci. 1989, 559, 37–55. [Google Scholar] [CrossRef]
- Yang, B.; Fritsche, K.L.; Beversdorf, D.Q.; Gu, Z.; Lee, J.C.; Folk, W.R.; Greenlief, C.M.; Sun, G.Y. Yin-Yang mechanisms regulating lipid peroxidation of docosahexaenoic acid and arachidonic acid in the central nervous system. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef]
- Shoji, H.; Ikeda, N.; Hosozawa, M.; Ohkawa, N.; Matsunaga, N.; Suganuma, H.; Hisata, K.; Tanaka, K.; Shimizu, T. Oxidative stress early in infancy and neurodevelopmental outcome in very low-birthweight infants. Pediatr. Int. 2014, 56, 709–713. [Google Scholar] [CrossRef]
- Bharadwaj, S.K.; Vishnu Bhat, B.; Vickneswaran, V.; Adhisivam, B.; Bobby, Z.; Habeebullah, S. Oxidative stress, antioxidant status and neurodevelopmental outcome in neonates born to pre-eclamptic mothers. Indian J. Pediatr. 2018, 85, 351–357. [Google Scholar] [CrossRef]
- Padilla, N.; Junque, C.; Figueras, F.; Sanz-Cortes, M.; Bargallo, N.; Arranz, A.; Donaire, A.; Figueras, J.; Gratacos, E. Differential vulnerability of gray matter and white matter to intrauterine growth restriction in preterm infants at 12 months corrected age. Brain Res. 2014, 1545, 1–11. [Google Scholar] [CrossRef]
- Jantzie, L.L.; Getsy, P.M.; Denson, J.L.; Firl, D.J.; Maxwell, J.R.; Rogers, D.A.; Wilson, C.G.; Robinson, S. Prenatal hypoxia–ischemia induces abnormalities in Ca3 microstructure, potassium chloride Co-transporter 2 expression and inhibitory tone. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Jantzie, L.L.; Robinson, S. Preclinical models of encephalopathy of prematurity. Dev. Neurosci. 2015, 37, 277–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coq, J.O.; Delcour, M.; Ogawa, Y.; Peyronnet, J.; Castets, F.; Turle-Lorenzo, N.; Montel, V.; Bodineau, L.; Cardot, P.; Brocard, C.; et al. Mild intrauterine hypoperfusion leads to lumbar and cortical hyperexcitability, spasticity, and muscle dysfunctions in rats: Implications for prematurity. Front. Neurol. 2018, 9, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delcour, M.; Russier, M.; Amin, M.; Baud, O.; Paban, V.; Barbe, M.F.; Coq, J.O. Impact of prenatal ischemia on behavior, cognitive abilities and neuroanatomy in adult rats with white matter damage. Behav. Brain Res. 2012, 232, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Ushida, T.; Kotani, T.; Tsuda, H.; Imai, K.; Nakano, T.; Hirako, S.; Ito, Y.; Li, H.; Mano, Y.; Wang, J.; et al. Molecular hydrogen ameliorates several characteristics of preeclampsia in the reduced uterine perfusion pressure (RUPP) rat model. Free Radic. Biol. Med. 2016, 101, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Kotani, T.; Ito, M.; Nagai, T.; Ichinohashi, Y.; Yamada, K.; Ohno, K.; Kikkawa, F.; Toyokuni, S. Maternal molecular hydrogen administration ameliorates rat fetal hippocampal damage caused by in utero ischemia-reperfusion. Free Radic. Biol. Med. 2014, 69, 324–330. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rains, M.E.; Muncie, C.B.; Pang, Y.; Fan, L.-W.; Tien, L.-T.; Ojeda, N.B. Oxidative Stress and Neurodevelopmental Outcomes in Rat Offspring with Intrauterine Growth Restriction Induced by Reduced Uterine Perfusion. Brain Sci. 2021, 11, 78. https://doi.org/10.3390/brainsci11010078
Rains ME, Muncie CB, Pang Y, Fan L-W, Tien L-T, Ojeda NB. Oxidative Stress and Neurodevelopmental Outcomes in Rat Offspring with Intrauterine Growth Restriction Induced by Reduced Uterine Perfusion. Brain Sciences. 2021; 11(1):78. https://doi.org/10.3390/brainsci11010078
Chicago/Turabian StyleRains, Marcelo E., Colin B. Muncie, Yi Pang, Lir-Wan Fan, Lu-Tai Tien, and Norma B. Ojeda. 2021. "Oxidative Stress and Neurodevelopmental Outcomes in Rat Offspring with Intrauterine Growth Restriction Induced by Reduced Uterine Perfusion" Brain Sciences 11, no. 1: 78. https://doi.org/10.3390/brainsci11010078