Solution-Based Synthesis and Processing of Metal Chalcogenides for Thermoelectric Applications
Abstract
:1. Introduction
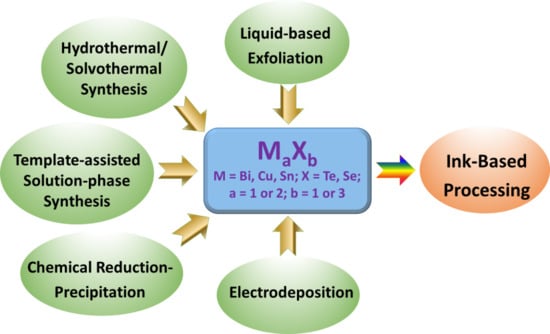
2. Solution-Based Approaches for Synthesis of Metal Chalcogenides
2.1. Liquid-Based Exfoliation Method
2.2. Hydrothermal or Solvothermal Method
2.3. Template-Assisted Solution-Phase Synthesis
2.4. Chemical Reduction–Precipitation Method
2.5. Electrodeposition Method
3. Ink-Based Processing Method for Thermoelectric Device Fabrication
4. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Minnich, A.J.; Dresselhaus, M.S.; Ren, Z.F.; Chen, G. Bulk nanostructured thermoelectric materials: Current research and future prospects. Energy Environ. Sci. 2009, 2, 466–479. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.G.; Lee, H.; Wang, D.Z.; Ren, Z.F.; Fleurial, J.P.; Gogna, P. New directions for low-dimensional thermoelectric materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.-D. Thermoelectric materials: Energy conversion between heat and electricity. J. Mater. 2015, 1, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Finefrock, S.W.; Yang, H.R.; Fang, H.Y.; Wu, Y. Thermoelectric Properties of Solution Synthesized Nanostructured Materials. In Annual Review of Chemical and Biomolecular Engineering; Prausnitz, J.M., Ed.; Annual Reviews: Palo Alto, CA, USA, 2015; Volume 6, pp. 247–266. [Google Scholar]
- Han, C.; Sun, Q.; Li, Z.; Dou, S.X. Thermoelectric enhancement of different kinds of metal chalcogenides. Adv. Energy Mater. 2016, 6, 1600498. [Google Scholar] [CrossRef]
- Chen, M.M.; Xue, H.G.; Guo, S.P. Multinary metal chalcogenides with tetrahedral structures for second-order nonlinear optical, photocatalytic, and photovoltaic applications. Coord. Chem. Rev. 2018, 368, 115–133. [Google Scholar] [CrossRef]
- Deng, Z.N.; Jiang, H.; Li, C.Z. 2D Metal Chalcogenides Incorporated into Carbon and their Assembly for Energy Storage Applications. Small 2018, 14, 9. [Google Scholar] [CrossRef]
- Guo, Z.N.; Sun, F.; Yuan, W.X. Chemical Intercalations in Layered Transition Metal Chalcogenides: Syntheses, Structures, and Related Properties. Cryst. Growth Des. 2017, 17, 2238–2253. [Google Scholar] [CrossRef]
- Jung, Y.; Zhou, Y.; Cha, J.J. Intercalation in two-dimensional transition metal chalcogenides. Inorg. Chem. Front. 2016, 3, 452–463. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Zhu, J.X.; Yan, Q.Y.; Dou, S.X.; Sun, W.P. Nanostructured Metal Chalcogenides for Energy Storage and Electrocatalysis. Adv. Funct. Mater. 2017, 27, 34. [Google Scholar] [CrossRef]
- Gao, M.-R.; Xu, Y.-F.; Jiang, J.; Yu, S.-H. Nanostructured metal chalcogenides: Synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 2013, 42, 2986–3017. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Karuppasamy, K.; Durai, G.; Rana, A.S.; Arunachalam, P.; Sangeetha, K.; Kuppusami, P.; Kim, H.S. Recent Advances in Metal Chalcogenides (MX; X = S, Se) Nanostructures for Electrochemical Supercapacitor Applications: A Brief Review. Nanomaterials 2018, 8, 256. [Google Scholar] [CrossRef]
- Xia, C.X.; Li, J.B. Recent advances in optoelectronic properties and applications of two-dimensional metal chalcogenides. J. Semicond. 2016, 37, 9. [Google Scholar] [CrossRef]
- Nie, L.N.; Zhang, Q.C. Recent progress in crystalline metal chalcogenides as efficient photocatalysts for organic pollutant degradation. Inorg. Chem. Front. 2017, 4, 1953–1962. [Google Scholar] [CrossRef]
- Gao, M.R.; Jiang, J.; Yu, S.H. Solution-based synthesis and design of late transition metal chalcogenide materials for oxygen reduction reaction (ORR). Small 2012, 8, 13–27. [Google Scholar] [CrossRef]
- Lhuillier, E.; Pedetti, S.; Ithurria, S.; Nadal, B.; Heuclin, H.; Dubertret, B. Two-Dimensional Colloidal Metal Chalcogenides Semiconductors: Synthesis, Spectroscopy, and Applications. Acc. Chem. Res. 2015, 48, 22–30. [Google Scholar] [CrossRef]
- Vaidhyanathan, B.; Ganguli, M.; Rao, K. Fast solid state synthesis of metal vanadates and chalcogenides using microwave irradiation. Mater. Res. Bull. 1995, 30, 1173–1177. [Google Scholar] [CrossRef]
- Carmalt, C.; Morrison, D.; Parkin, I. Solid-state and solution phase metathetical synthesis of copper indium chalcogenides. J. Mater. Chem. 1998, 8, 2209–2211. [Google Scholar] [CrossRef]
- Shaw, G.A.; Morrison, D.E.; Parkin, I.P. Solid state synthesis of binary metal chalcogenides. J. Chem. Soc. Dalton Trans. 2001, 1872–1875. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Yin, Z.; Zhang, H. Preparation and applications of mechanically exfoliated single-layer and multilayer MoS2 and WSe2 nanosheets. Acc. Chem. Res. 2014, 47, 1067–1075. [Google Scholar] [CrossRef]
- Desai, S.B.; Madhvapathy, S.R.; Amani, M.; Kiriya, D.; Hettick, M.; Tosun, M.; Zhou, Y.; Dubey, M.; Ager, J.W., III; Chrzan, D. Gold-Mediated Exfoliation of Ultralarge Optoelectronically-Perfect Monolayers. Adv. Mater. 2016, 28, 4053–4058. [Google Scholar] [CrossRef]
- Magda, G.Z.; Pető, J.; Dobrik, G.; Hwang, C.; Biró, L.P.; Tapasztó, L. Exfoliation of large-area transition metal chalcogenide single layers. Sci. Rep. 2015, 5, 14714. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Venkatasubramanian, R.; Liu, C.; Pierce, J.; Yang, H.; Zahid Hasan, M.; Wu, Y.; Chen, Y.P. Topological insulator Bi2Te3 films synthesized by metal organic chemical vapor deposition. Appl. Phys. Lett. 2012, 101, 162104. [Google Scholar] [CrossRef]
- Lee, C.W.; Kim, G.H.; Kang, S.G.; Kang, M.A.; An, K.S.; Kim, H.; Lee, Y.K. Growth behavior of Bi2Te3 and Sb2Te3 thin films on graphene substrate grown by plasma-enhanced chemical vapor deposition. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2017, 11, 1600369. [Google Scholar] [CrossRef]
- Yu, J.; Hu, X.; Li, H.; Zhou, X.; Zhai, T. Large-scale synthesis of 2D metal dichalcogenides. J. Mater. Chem. C 2018, 6, 4627–4640. [Google Scholar] [CrossRef]
- Guo, L.L.; Ivey, B.C.; Aglan, A.; Tang, C.L.; Song, J.H.; Turner, C.H.; Frazier, R.M.; Gupta, A.; Wang, H.T. Vapor Phase Growth of Bismuth Telluride Nanoplatelets on Flexible Polyimide Films. ECS Solid State Lett. 2013, 2, P19–P21. [Google Scholar] [CrossRef]
- Tung, Y.; Chiang, Y.; Chong, C.; Deng, Z.; Chen, Y.; Huang, J.; Cheng, C.-M.; Pi, T.-W.; Tsuei, K.-D.; Li, Z. Growth and characterization of molecular beam epitaxy-grown Bi2Te3− xSex topological insulator alloys. J. Appl. Phys. 2016, 119, 055303. [Google Scholar] [CrossRef]
- Zeng, Z.; Morgan, T.A.; Fan, D.; Li, C.; Hirono, Y.; Hu, X.; Zhao, Y.; Lee, J.S.; Wang, J.; Wang, Z.M. Molecular beam epitaxial growth of Bi2Te3 and Sb2Te3 topological insulators on GaAs (111) substrates: A potential route to fabricate topological insulator p-n junction. AIP Adv. 2013, 3, 072112. [Google Scholar] [CrossRef]
- Tan, C.L.; Cao, X.H.; Wu, X.J.; He, Q.Y.; Yang, J.; Zhang, X.; Chen, J.Z.; Zhao, W.; Han, S.K.; Nam, G.H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, K. Recent progress on graphene-analogous 2D nanomaterials: Properties, modeling and applications. Prog. Mater Sci. 2019, 100, 99–169. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Dyck, J.S.; Burda, C. Toward high-performance nanostructured thermoelectric materials: The progress of bottom-up solution chemistry approaches. J. Mater. Chem. 2011, 21, 17049–17058. [Google Scholar] [CrossRef]
- Velický, M.; Toth, P.S. From two-dimensional materials to their heterostructures: An electrochemist’s perspective. Appl. Mater. Today 2017, 8, 68–103. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, L.D. Promising thermoelectric bulk materials with 2D structures. Adv. Mater. 2017, 29, 1702676. [Google Scholar] [CrossRef]
- Zhao, Y.; Hughes, R.W.; Su, Z.; Zhou, W.; Gregory, D.H. One-Step Synthesis of Bismuth Telluride Nanosheets of a Few Quintuple Layers in Thickness. Angew. Chem. Int. Ed. 2011, 50, 10397–10401. [Google Scholar] [CrossRef]
- Kong, D.S.; Dang, W.H.; Cha, J.J.; Li, H.; Meister, S.; Peng, H.L.; Liu, Z.F.; Cui, Y. Few-Layer Nanoplates of Bi2Se3 and Bi2Te3 with Highly Tunable Chemical Potential. Nano Lett. 2010, 10, 2245–2250. [Google Scholar] [CrossRef]
- Fei, F.; Wei, Z.; Wang, Q.; Lu, P.; Wang, S.; Qin, Y.; Pan, D.; Zhao, B.; Wang, X.; Sun, J. Solvothermal synthesis of lateral heterojunction Sb2Te3/Bi2Te3 nanoplates. Nano Lett. 2015, 15, 5905–5911. [Google Scholar] [CrossRef]
- Teweldebrhan, D.; Goyal, V.; Balandin, A.A. Exfoliation and characterization of bismuth telluride atomic quintuples and quasi-two-dimensional crystals. Nano Lett. 2010, 10, 1209–1218. [Google Scholar] [CrossRef]
- Backes, C.; Higgins, T.M.; Kelly, A.; Boland, C.; Harvey, A.; Hanlon, D.; Coleman, J.N. Guidelines for exfoliation, characterization and processing of layered materials produced by liquid exfoliation. Chem. Mater. 2016, 29, 243–255. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Cao, H.Z.; Xue, Y.Q.; Li, B.; Cai, W.H. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef]
- Hong, S.S.; Kundhikanjana, W.; Cha, J.J.; Lai, K.; Kong, D.; Meister, S.; Kelly, M.A.; Shen, Z.-X.; Cui, Y. Ultra-thin Topological Insulator Bi2Se3 Nanoribbons Exfoliated by Atomic Force Microscopy. Nano Lett. 2010, 10, 3118–3122. [Google Scholar] [CrossRef]
- Ding, Z.; Bux, S.K.; King, D.J.; Chang, F.L.; Chen, T.-H.; Huang, S.-C.; Kaner, R.B. Lithium intercalation and exfoliation of layered bismuth selenide and bismuth telluride. J. Mater. Chem. 2009, 19, 2588–2592. [Google Scholar] [CrossRef]
- Ju, H.; Kim, M.; Park, D.; Kim, J. A Strategy for Low Thermal Conductivity and Enhanced Thermoelectric Performance in SnSe: Porous SnSe1–x S x Nanosheets. Chem. Mater. 2017, 29, 3228–3236. [Google Scholar] [CrossRef]
- Ludwig, T.; Guo, L.; McCrary, P.; Zhang, Z.; Gordon, H.; Quan, H.; Stanton, M.; Frazier, R.M.; Rogers, R.D.; Wang, H.-T. Mechanism of bismuth telluride exfoliation in an ionic liquid solvent. Langmuir 2015, 31, 3644–3652. [Google Scholar] [CrossRef]
- Abedini, A.; Ludwig, T.; Zhang, Z.; Turner, C.H. Molecular Dynamics Simulation of Bismuth Telluride Exfoliation Mechanisms in Different Ionic Liquid Solvents. Langmuir 2016, 32, 9982–9992. [Google Scholar] [CrossRef]
- Yang, Y.C.; Hou, H.S.; Zou, G.Q.; Shi, W.; Shuai, H.L.; Li, J.Y.; Ji, X.B. Electrochemical exfoliation of graphene-like two-dimensional nanomaterials. Nanoscale 2019, 11, 16–33. [Google Scholar] [CrossRef]
- Ambrosi, A.; Sofer, Z.k.; Luxa, J.; Pumera, M. Exfoliation of layered topological insulators Bi2Se3 and Bi2Te3 via electrochemistry. ACS Nano 2016, 10, 11442–11448. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Song, S.; Zhang, H. Hydrothermal synthetic strategies of inorganic semiconducting nanostructures. Chem. Soc. Rev. 2013, 42, 5714–5743. [Google Scholar] [CrossRef]
- Mi, J.L.; Lock, N.; Sun, T.; Christensen, M.; Sondergaard, M.; Hald, P.; Hng, H.H.; Ma, J.; Iversen, B.B. Biomolecule-Assisted Hydrothermal Synthesis and Self-Assembly of Bi2Te3 Nanostring-Cluster Hierarchical Structure. ACS Nano 2010, 4, 2523–2530. [Google Scholar] [CrossRef]
- Kundu, S.; Yi, S.I.; Yu, C. Gram-scale solution-based synthesis of SnSe thermoelectric nanomaterials. Appl. Surf. Sci. 2018, 459, 376–384. [Google Scholar] [CrossRef]
- Song, S.Y.; Fu, J.P.; Li, X.Y.; Gao, W.; Zhang, H.J. Facile Synthesis and Thermoelectric Properties of Self-assembled Bi2Te3 One-Dimensional Nanorod Bundles. Chem. A Eur. J. 2013, 19, 2889–2894. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.G. A facile two-step hydrothermal route for the synthesis of low-dimensional structured Bi2Te3 nanocrystals with various morphologies. J. Alloy. Compd. 2010, 497, 57–61. [Google Scholar] [CrossRef]
- Guo, J.; Jian, J.K.; Liu, J.; Cao, B.L.; Lei, R.B.; Zhang, Z.H.; Song, B.; Zhao, H.Z. Synthesis of SnSe nanobelts and the enhanced thermoelectric performance in its hot-pressed bulk composite. Nano Energy 2017, 38, 569–575. [Google Scholar] [CrossRef]
- Fu, J.P.; Song, S.Y.; Zhang, X.G.; Cao, F.; Zhou, L.; Li, X.Y.; Zhang, H.J. Bi2Te3 nanoplates and nanoflowers: Synthesized by hydrothermal process and their enhanced thermoelectric properties. CrystEngComm 2012, 14, 2159–2165. [Google Scholar] [CrossRef]
- Hong, M.; Chasapis, T.C.; Chen, Z.G.; Yang, L.; Kanatzidis, M.G.; Snyder, G.J.; Zou, J. n-Type Bi2Te3-xSex Nanoplates with Enhanced Thermoelectric Efficiency Driven by Wide-Frequency Phonon Scatterings and Synergistic Carrier Scatterings. ACS Nano 2016, 10, 4719–4727. [Google Scholar] [CrossRef]
- Li, C.C.; Kong, F.F.; Liu, C.C.; Liu, H.X.; Hu, Y.J.; Wang, T.Z.; Xu, J.K.; Jiang, F.X. Dual-functional aniline-assisted wet-chemical synthesis of bismuth telluride nanoplatelets and their thermoelectric performance. Nanotechnology 2017, 28, 9. [Google Scholar] [CrossRef]
- Shi, X.L.; Chen, Z.G.; Liu, W.D.; Yang, L.; Hong, M.; Moshwan, R.; Huang, L.Q.; Zou, J. Achieving high Figure of Merit in p-type polycrystalline Sn0.98Se via self-doping and anisotropy-strengthening. Energy Storage Mater. 2018, 10, 130–138. [Google Scholar] [CrossRef]
- Shi, X.L.; Zheng, K.; Liu, W.D.; Wang, Y.; Yang, Y.Z.; Chen, Z.G.; Zou, J. Realizing High Thermoelectric Performance in n-Type Highly Distorted Sb-Doped SnSe Microplates via Tuning High Electron Concentration and Inducing Intensive Crystal Defects. Adv. Energy Mater. 2018, 8, 12. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Miao, N.; Zhou, J.; Sun, Z. I-doped Cu2Se nanocrystals for high-performance thermoelectric applications. J. Alloy. Compd. 2019, 772, 366–370. [Google Scholar] [CrossRef]
- Wu, F.; Song, H.Z.; Jia, J.F.; Hu, X. Effects of Ce, Y, and Sm doping on the thermoelectric properties of Bi2Te3 alloy. Prog. Nat. Sci. Mater. Int. 2013, 23, 408–412. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.G.; Han, G.; Hong, M.; Zou, J. Impacts of Cu deficiency on the thermoelectric properties of Cu2-XSe nanoplates. Acta Mater. 2016, 113, 140–146. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhu, T.J.; Tu, J.P.; Zhao, X.B. Flower-like nanostructure and thermoelectric properties of hydrothermally synthesized La-containing Bi2Te3 based alloys. Mater. Chem. Phys. 2007, 103, 484–488. [Google Scholar] [CrossRef]
- Shi, W.; Wu, F.; Wang, K.; Yang, J.; Song, H.; Hu, X. Preparation and thermoelectric properties of yttrium-doped Bi2Te3 flower-like nanopowders. J. Electron. Mater. 2014, 43, 3162–3168. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Bazarganipour, M.; Davar, F. Hydrothermal preparation and characterization of based-alloy Bi2Te3 nanostructure with different morphology. J. Alloy. Compd. 2010, 489, 530–534. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Ge, Z.H.; Feng, J. Synthesis and Thermoelectric Properties of Copper Sulfides via Solution Phase Methods and Spark Plasma Sintering. Crystals 2017, 7, 141. [Google Scholar] [CrossRef]
- Wang, K.; Liang, H.-W.; Yao, W.-T.; Yu, S.-H. Templating synthesis of uniform Bi2Te3 nanowires with high aspect ratio in triethylene glycol (TEG) and their thermoelectric performance. J. Mater. Chem. 2011, 21, 15057–15062. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Kirk, B.; Jauregui, L.A.; Yang, H.R.; Xu, X.F.; Chen, Y.P.; Wu, Y. Rational Synthesis of Ultrathin n-Type Bi2Te3 Nanowires with Enhanced Thermoelectric Properties. Nano Lett. 2012, 12, 56–60. [Google Scholar] [CrossRef]
- Han, C.; Li, Z.; Lu, G.Q.; Dou, S.X. Robust scalable synthesis of surfactant-free thermoelectric metal chalcogenide nanostructures. Nano Energy 2015, 15, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Qin, X.Y.; Liu, Y.F.; Song, C.J.; Wang, L.; Zhang, J.; Xin, H.X.; Guo, G.L.; Zou, T.H.; Sun, G.L. Chemical synthesis of nanostructured Cu2Se with high thermoelectric performance. Rsc Adv. 2014, 4, 8638–8644. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chen, Y.-Y.; Lin, S.-J.; Ho, J.C.; Lee, P.-C.; Chen, C.-D.; Harutyunyan, S.R. Fabrication and characterization of electrodeposited bismuth telluride films and nanowires. J. Phys. Chem. C 2010, 114, 3385–3389. [Google Scholar] [CrossRef]
- Manzano, C.V.; Abad, B.; Rojo, M.M.; Koh, Y.R.; Hodson, S.L.; Martinez, A.M.L.; Xu, X.F.; Shakouri, A.; Sands, T.D.; Borca-Tasciuc, T.; et al. Anisotropic Effects on the Thermoelectric Properties of Highly Oriented Electrodeposited Bi2Te3 Films. Sci. Rep. 2016, 6, 8. [Google Scholar] [CrossRef]
- Moon, G.D.; Ko, S.; Xia, Y.; Jeong, U. Chemical transformations in ultrathin chalcogenide nanowires. ACS Nano 2010, 4, 2307–2319. [Google Scholar] [CrossRef]
- Moon, G.D.; Ko, S.; Min, Y.; Zeng, J.; Xia, Y.; Jeong, U. Chemical transformations of nanostructured materials. Nano Today 2011, 6, 186–203. [Google Scholar] [CrossRef]
- Liang, H.W.; Liu, S.; Yu, S.H. Controlled synthesis of one-dimensional inorganic nanostructures using pre-existing one-dimensional nanostructures as templates. Adv. Mater. 2010, 22, 3925–3937. [Google Scholar] [CrossRef]
- Finefrock, S.W.; Fang, H.; Yang, H.; Darsono, H.; Wu, Y. Large-scale solution-phase production of Bi2Te3 and PbTe nanowires using Te nanowire templates. Nanoscale 2014, 6, 7872–7876. [Google Scholar] [CrossRef]
- Pejjai, B.; Reddy, V.R.M.; Seku, K.; Pallavolu, M.R.; Park, C. Eco-friendly synthesis of SnSe nanoparticles: Effect of reducing agents on the reactivity of a Se-precursor and phase formation of SnSe NPs. New J. Chem. 2018, 42, 4843–4853. [Google Scholar] [CrossRef]
- Gurrappa, I.; Binder, L. Electrodeposition of nanostructured coatings and their characterization—a review. Sci. Technol. Adv. Mater. 2008, 9, 043001. [Google Scholar] [CrossRef]
- Rostek, R.; Stein, N.; Boulanger, C. A review of electroplating for V-VI thermoelectric films: From synthesis to device integration. J. Mater. Res. 2015, 30, 2518–2543. [Google Scholar] [CrossRef]
- Bicelli, L.P.; Bozzini, B.; Mele, C.; D’Urzo, L. A review of nanostructural aspects of metal electrodeposition. Int. J. Electrochem. Sci 2008, 3, 356–408. [Google Scholar]
- Menke, E.; Li, Q.; Penner, R. Bismuth telluride (Bi2Te3) nanowires synthesized by cyclic electrodeposition/stripping coupled with step edge decoration. Nano Lett. 2004, 4, 2009–2014. [Google Scholar] [CrossRef]
- Wang, W.-L.; Wan, C.-C.; Wang, Y.-Y. Composition-dependent characterization and optimal control of electrodeposited Bi2Te3 films for thermoelectric application. Electrochim. Acta 2007, 52, 6502–6508. [Google Scholar] [CrossRef]
- Heo, P.; Hagiwara, K.; Ichino, R.; Okido, M. Electrodeposition and thermoelectric characterization of Bi2Te3. J. Electrochem. Soc. 2006, 153, C213–C217. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Wu, M.X.; Su, J.L.; Vullers, R.J.M.; Vereecken, P.M.; Fransaer, J. Electrodeposition of bismuth telluride thermoelectric films from a nonaqueous electrolyte using ethylene glycol. Electrochim. Acta 2012, 68, 9–17. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Kajitani, T. Preparation of Bi2Te3 films by electrodeposition. J. Cryst. Growth 2001, 229, 542–546. [Google Scholar] [CrossRef]
- Naylor, A.J.; Koukharenko, E.; Nandhakumar, I.S.; White, N.M. Surfactant-Mediated Electrodeposition of Bismuth Telluride Films and Its Effect on Microstructural Properties. Langmuir 2012, 28, 8296–8299. [Google Scholar] [CrossRef]
- Ma, Y.; Ahlberg, E.; Sun, Y.; Iversen, B.B.; Palmqvist, A.E. Thermoelectric properties of thin films of bismuth telluride electrochemically deposited on stainless steel substrates. Electrochim. Acta 2011, 56, 4216–4223. [Google Scholar] [CrossRef]
- Yoo, B.Y.; Huang, C.K.; Lim, J.R.; Herman, J.; Ryan, M.A.; Fleurial, J.P.; Myung, N.V. Electrochemically deposited thermoelectric n-type Bi2Te3 thin films. Electrochim. Acta 2005, 50, 4371–4377. [Google Scholar] [CrossRef]
- Prieto, A.L.; Sander, M.S.; Martín-González, M.S.; Gronsky, R.; Sands, T.; Stacy, A.M. Electrodeposition of ordered Bi2Te3 nanowire arrays. J. Am. Chem. Soc. 2001, 123, 7160–7161. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Huang, X.; Li, G.; Zhang, L. Pulsed electrodeposition of single-crystalline Bi2Te3 nanowire arrays. Nanotechnology 2006, 17, 1706. [Google Scholar] [CrossRef]
- Lee, J.; Farhangfar, S.; Lee, J.; Cagnon, L.; Scholz, R.; Gösele, U.; Nielsch, K. Tuning the crystallinity of thermoelectric Bi2Te3 nanowire arrays grown by pulsed electrodeposition. Nanotechnology 2008, 19, 365701. [Google Scholar] [CrossRef]
- Sander, M.; Prieto, A.; Gronsky, R.; Sands, T.; Stacy, A.M. Fabrication of High-Density, High Aspect Ratio, Large-Area Bismuth Telluride Nanowire Arrays by Electrodeposition into Porous Anodic Alumina Templates. Adv. Mater. 2002, 14, 665–667. [Google Scholar] [CrossRef]
- Li, X.-H.; Zhou, B.; Pu, L.; Zhu, J.-J. Electrodeposition of Bi2Te3 and Bi2Te3 derived alloy nanotube arrays. Cryst. Growth Des. 2008, 8, 771–775. [Google Scholar] [CrossRef]
- Yang, M.Q.; Shen, Z.W.; Liu, X.Q.; Wang, W. Electrodeposition and Thermoelectric Properties of Cu-Se Binary Compound Films. J. Electron. Mater. 2016, 45, 1974–1981. [Google Scholar] [CrossRef]
- Lippkow, D.; Strehblow, H.-H. Structural investigations of thin films of copper–selenide electrodeposited at elevated temperatures. Electrochim. Acta 1998, 43, 2131–2140. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, J.; Singh, L.; Singh, S. Electrochemical synthesis and characterization of Cu2Se nanowires. Superlattices Microstruct. 2013, 64, 294–302. [Google Scholar] [CrossRef]
- Ananthi, K.; Thilakavathy, K.; Muthukumarasamy, N.; Dhanapandian, S.; Murali, K. Properties of pulse plated SnSe films. J. Mater. Sci.: Mater. Electron. 2012, 23, 1338–1341. [Google Scholar] [CrossRef]
- Bicer, M.; Şişman, İ. Electrodeposition and growth mechanism of SnSe thin films. Appl. Surf. Sci. 2011, 257, 2944–2949. [Google Scholar] [CrossRef]
- Mathews, N. Electrodeposited tin selenide thin films for photovoltaic applications. Sol. Energy 2012, 86, 1010–1016. [Google Scholar] [CrossRef]
- Zainal, Z.; Ali, A.J.; Kassim, A.; Hussein, M.Z. Electrodeposition of tin selenide thin film semiconductor: Effect of the electrolytes concentration on the film properties. Sol. Energy Mater. Sol. Cells 2003, 79, 125–132. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Oh, T.-S. Thermoelectric power generation characteristics of a thin-film device consisting of electrodeposited n-Bi2Te3 and p-Sb2Te3 thin-film legs. J. Electron. Mater. 2013, 42, 2752–2757. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, C.; Song, H.; Xu, J.; Mo, D.; Shi, H.; Wang, Z.; Jiang, F.; Lu, B.; Zhu, Z. Free-standing PEDOT: PSS film as electrode for the electrodeposition of bismuth telluride and its thermoelectric performance. Int. J. Electrochem. Sci 2014, 9, 7540–7551. [Google Scholar]
- Larson, C.; Farr, J. Recent advances in pulsed current electrodeposition: A brief review. Trans. IMF 2010, 88, 237–242. [Google Scholar] [CrossRef]
- Jo, S.; Choo, S.; Kim, F.; Heo, S.H.; Son, J.S. Ink Processing for Thermoelectric Materials and Power-Generating Devices. Adv. Mater. 2018, 1804930. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. Thermoelectric energy from flexible P3HT films doped with a ferric salt of triflimide anions. Energy Environ. Sci. 2012, 5, 9639–9644. [Google Scholar] [CrossRef]
- Hwang, S.; Potscavage, W.J.; Yang, Y.S.; Park, I.S.; Matsushima, T.; Adachi, C. Solution-processed organic thermoelectric materials exhibiting doping-concentration-dependent polarity. Phys. Chem. Chem. Phys. 2016, 18, 29199–29207. [Google Scholar] [CrossRef]
- Taggart, D.K.; Yang, Y.; Kung, S.-C.; McIntire, T.M.; Penner, R.M. Enhanced thermoelectric metrics in ultra-long electrodeposited PEDOT nanowires. Nano Lett. 2010, 11, 125–131. [Google Scholar] [CrossRef]
- Kim, G.-H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719. [Google Scholar] [CrossRef]
- Aïch, R.B.; Blouin, N.; Bouchard, A.; Leclerc, M. Electrical and thermoelectric properties of poly (2, 7-carbazole) derivatives. Chem. Mater. 2009, 21, 751–757. [Google Scholar] [CrossRef]
- Jiang, F.; Xiong, J.; Zhou, W.; Liu, C.; Wang, L.; Zhao, F.; Liu, H.; Xu, J. Use of organic solvent-assisted exfoliated MoS 2 for optimizing the thermoelectric performance of flexible PEDOT: PSS thin films. J. Mater. Chem. A 2016, 4, 5265–5273. [Google Scholar] [CrossRef]
- Ju, H.; Kim, J. Chemically exfoliated SnSe nanosheets and their SnSe/poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) composite films for polymer based thermoelectric applications. ACS Nano 2016, 10, 5730–5739. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.Y.; Lee, S.S.; Park, C.R.; Kim, H. High-Performance Thermoelectric Paper Based on Double Carrier-Filtering Processes at Nanowire Heterojunctions. Adv. Energy Mater. 2016, 6, 1502181. [Google Scholar] [CrossRef]
- Kato, K.; Hagino, H.; Miyazaki, K. Fabrication of bismuth telluride thermoelectric films containing conductive polymers using a printing method. J. Electron. Mater. 2013, 42, 1313–1318. [Google Scholar] [CrossRef]
- Toshima, N.; Imai, M.; Ichikawa, S. Organic–inorganic nanohybrids as novel thermoelectric materials: Hybrids of polyaniline and bismuth (III) telluride nanoparticles. J. Electron. Mater. 2011, 40, 898–902. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, J.; Katz, H.; Fang, F.; Opila, R. Promising thermoelectric properties of commercial PEDOT: PSS materials and their Bi2Te3 powder composites. ACS Appl. Mater. Interfaces 2010, 2, 3170–3178. [Google Scholar] [CrossRef]
- Lin, Z.; Hollar, C.; Kang, J.S.; Yin, A.; Wang, Y.; Shiu, H.Y.; Huang, Y.; Hu, Y.; Zhang, Y.; Duan, X. A Solution Processable High-Performance Thermoelectric Copper Selenide Thin Film. Adv. Mater. 2017, 29, 1606662. [Google Scholar] [CrossRef]
- Kim, S.J.; We, J.H.; Cho, B.J. A wearable thermoelectric generator fabricated on a glass fabric. Energy Environ. Sci. 2014, 7, 1959–1965. [Google Scholar] [CrossRef]
- Choi, H.; Kim, S.J.; Kim, Y.; We, J.H.; Oh, M.-W.; Cho, B.J. Enhanced thermoelectric properties of screen-printed Bi0.5Sb1.5Te3 and Bi2Te2.7Se0.3 thick films using a post annealing process with mechanical pressure. J. Mater. Chem. C 2017, 5, 8559–8565. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, H.; Kim, Y.; We, J.H.; Shin, J.S.; Lee, H.E.; Oh, M.-W.; Lee, K.J.; Cho, B.J. Post ionized defect engineering of the screen-printed Bi2Te2.7Se0.3 thick film for high performance flexible thermoelectric generator. Nano Energy 2017, 31, 258–263. [Google Scholar] [CrossRef]
- Choi, H.; Kim, Y.J.; Kim, C.S.; Yang, H.M.; Oh, M.W.; Cho, B.J. Enhancement of reproducibility and reliability in a high-performance flexible thermoelectric generator using screen-printed materials. Nano Energy 2018, 46, 39–44. [Google Scholar] [CrossRef]
- Park, S.H.; Jo, S.; Kwon, B.; Kim, F.; Ban, H.W.; Lee, J.E.; Gu, D.H.; Lee, S.H.; Hwang, Y.; Kim, J.-S. High-performance shape-engineerable thermoelectric painting. Nat. Commun. 2016, 7, 13403. [Google Scholar] [CrossRef] [Green Version]
- Kim, F.; Kwon, B.; Eom, Y.; Lee, J.E.; Park, S.; Jo, S.; Park, S.H.; Kim, B.-S.; Im, H.J.; Lee, M.H. 3D printing of shape-conformable thermoelectric materials using all-inorganic Bi2Te3-based inks. Nat. Energy 2018, 3, 301. [Google Scholar] [CrossRef]
- We, J.H.; Kim, S.J.; Kim, G.S.; Cho, B.J. Improvement of thermoelectric properties of screen-printed Bi2Te3 thick film by optimization of the annealing process. J. Alloy. Compd. 2013, 552, 107–110. [Google Scholar] [CrossRef]
- Chen, B.; Das, S.R.; Zheng, W.; Zhu, B.; Xu, B.; Hong, S.; Sun, C.; Wang, X.; Wu, Y.; Claussen, J.C. Inkjet Printing of Single-Crystalline Bi2Te3 Thermoelectric Nanowire Networks. Adv. Electron. Mater. 2017, 3, 1600524. [Google Scholar] [CrossRef]
- Orrill, M.; LeBlanc, S. Printed thermoelectric materials and devices: Fabrication techniques, advantages, and challenges. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Madan, D.; Wang, Z.; Chen, A.; Juang, R.-C.; Keist, J.; Wright, P.K.; Evans, J.W. Enhanced performance of dispenser printed MA n-type Bi2Te3 composite thermoelectric generators. ACS Appl. Mater. Interfaces 2012, 4, 6117–6124. [Google Scholar] [CrossRef]
- Chen, A.; Madan, D.; Mahlstedt, B.; Wright, P.; Evans, J. Dispenser-printed thick film thermoelectric materials. In Proceedings of the PowerMEMS, Leuven, Belgium, 30 November 2010; pp. 223–226. [Google Scholar]
- Cao, Z.; Koukharenko, E.; Tudor, M.; Torah, R.; Beeby, S. Flexible screen printed thermoelectric generator with enhanced processes and materials. Sens. Actuators A Phys. 2016, 238, 196–206. [Google Scholar] [CrossRef]
- Madan, D.; Chen, A.; Wright, P.K.; Evans, J.W. Printed Se-doped MA n-type Bi2Te3 thick-film thermoelectric generators. J. Electron. Mater. 2012, 41, 1481–1486. [Google Scholar] [CrossRef]
- Chen, A.; Madan, D.; Wright, P.; Evans, J. Dispenser-printed planar thick-film thermoelectric energy generators. J. Micromech. Microeng. 2011, 21, 104006. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Spokoyny, B.; Lee, J.-S.; Scheele, M.; Weber, A.; Perera, S.; Landry, D.; Talapin, D.V. Semiconductor nanocrystals functionalized with antimony telluride zintl ions for nanostructured thermoelectrics. J. Am. Chem. Soc. 2010, 132, 6686–6695. [Google Scholar] [CrossRef]
- Han, C.; Li, Z.; Dou, S. Recent progress in thermoelectric materials. Chin. Sci. Bull. 2014, 59, 2073–2091. [Google Scholar] [CrossRef]












| Materials | Approach | σ (S cm−1) | S (µV K−1) | κ (W m−1 K−1) | ZTmax | T (K) | Ref. |
|---|---|---|---|---|---|---|---|
| Bi2Te3 nanostrings | HT + HP | 5.1 | −172 | 0.29 | 0.016 | 298 | [51] |
| Y-doped Bi2Te3 nanopowders | HT + HP | 1.180 | −150 | 0.85 | 1.23 | 410 | [65] |
| Bi2Te3 nanoflowers | HT + PS | 370 | −152 | 0.5 | 0.7 | 453 | [56] |
| La-doped Bi2Te3 nanoflowers | HT + HP | 62 | −133 | 0.92 | 0.58 | 480 | [64] |
| Ce-doped Bi2Te3 nanosheets | HT + HP | 770 | −146 | 0.5 | 1.29 | 398 | [62] |
| Cu2Se nanoplates | HT + SPS | 1000 | 101 | 1.2 | 0.62 | 773 | [67] |
| I-doped Cu2Se nanosheets | HT + HP | 250 | 215 | 0.8 | 1.1 | 773 | [61] |
| Bi2Te3 nanorods | ST + HP | 180 | −135 | 0.35 | 0.43 | 473 | [53] |
| Se-doped Bi2Te3 nanoplates | ST + SPS | 480 | −198 | 0.72 | 1.23 | 480 | [57] |
| Cu2Se nanoplates | ST + SPS | 100 | 290 | 0.3 | 1.82 | 850 | [63] |
| Sb-doped SnSe microplates | ST + SPS | 39.4 | 247 | 0.17 | 1.1 | 773 | [60] |
| SnSe nanobelts | ST + HP | 22.2 | 350 | 0.25 | 0.83 | 803 | [55] |
| Bi2Te3 nanowires | TA + HT | 13.3 | −74 | 0.05 | 0.005 | 300 | [68] |
| Bi2Te3 nanowires | TA + SPS | 425 | −250 | 1.0 | 0.96 | 380 | [69] |
| Cu2Se nanoparticles | CRP + SPS | 3000 | 37.5 | 0.75 | 0.3 | 673 | [70] |
| SnSe nanoparticles | CRP + SPS | 30 | −305 | 0.5 | 0.4 | 573 | [70] |
| Cu2Se nanopowders | CRP + HT | 333 | 140 | 0.75 | 1.35 | 900 | [71] |
| Bi2Te3 nanowires | ED + HT | 2000 | −70 | 0.75 | 0.9 | 300 | [72] |
| Bi2Te3 films | ED | 666 a 3200 b | −58 a −50 b | 1.2 a 2.4 b | 0.056 a 0.104 b | 300 | [73] |
| S-doped SnSe nanosheets | LE | 4.76 | 560 | 0.4 | 0.12 | 310 | [44] |
| Ink Formulation | Process | σ (S cm−1) | S (µV K−1) | κ (W m−1K−1) | Power Factor (μW m−1 K−2) | ZT | Power Density (µW cm−2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Bi2Te3/Sb2Te3 ChaMa | 3D printing | 330 | 190 | 0.54 | — | 0.9 | 1620 ΔT 39K | [123] |
| Bi2Te3/organic binder | Screen printing | 250 | 136 | 1 | 2100 | 0.61 | — | [124] |
| Bi2Te3/epoxy | Dispenser printing | 100 | 170 | 0.38 | 275 | 0.31 | 130 ΔT 20 K | [127] |
| Bi2Te3, Sb2Te3/organic binder | Screen printing | 670/1500 | 141/98 | 1.25/1 | 1332/1441 | 0.33/0.28 | 3800 ΔT 50 K | [118] |
| Bi2Te3, Sb2Te3/epoxy | Dispenser printing | 100/130 | 180/120 | 0.24/0.42 | 200/520 | 0.25/0.37 | 23 ΔT 10 K | [128] |
| Bi2Te3/epoxy | Screen printing | 100 | 135 | — | 1.05 | — | 1.11 ΔT 20 K | [129] |
| Bi2Te3 nanowires | Inkjet printing | 180 | 140 | — | 163 | 0.26 | — | [125] |
| Bi2Te3/epoxy | Dispenser printing | 400 | 200 | 0.27 | 1500 | 0.17 | 25 ΔT 20 K | [130] |
| Cu2Se | Spin coating | 500 | 111 | — | 620 | — | — | [117] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, K.W.; Wang, S.-X.; Zheng, Y.; Xu, J. Solution-Based Synthesis and Processing of Metal Chalcogenides for Thermoelectric Applications. Appl. Sci. 2019, 9, 1511. https://doi.org/10.3390/app9071511
Shah KW, Wang S-X, Zheng Y, Xu J. Solution-Based Synthesis and Processing of Metal Chalcogenides for Thermoelectric Applications. Applied Sciences. 2019; 9(7):1511. https://doi.org/10.3390/app9071511
Chicago/Turabian StyleShah, Kwok Wei, Su-Xi Wang, Yun Zheng, and Jianwei Xu. 2019. "Solution-Based Synthesis and Processing of Metal Chalcogenides for Thermoelectric Applications" Applied Sciences 9, no. 7: 1511. https://doi.org/10.3390/app9071511