Quantitative CT Analysis for Predicting the Behavior of Part-Solid Nodules with Solid Components Less than 6 mm: Size, Density and Shape Descriptors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient and SSN Selection
2.2. CT Acquisition
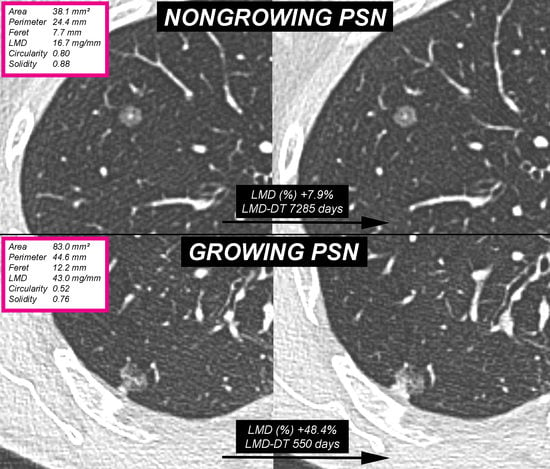
2.3. Computerized Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Truong, M.T.; Ko, J.P.; Rossi, S.E.; Rossi, I.; Viswanathan, C.; Bruzzi, J.F.; Marom, E.M.; Erasmus, J.J. Update in the evaluation of the solitary pulmonary nodule. Radiographics 2014, 34, 1658–1679. [Google Scholar] [CrossRef] [PubMed]
- Papapietro, V.R.; Milanese, G.; Borghesi, A.; Sverzellati, N.; Silva, M. Look around your target: A new approach to early diagnosis of lung cancer. Ann. Transl. Med. 2018, 6, S77. [Google Scholar] [CrossRef] [PubMed]
- Larici, A.R.; Farchione, A.; Franchi, P.; Ciliberto, M.; Cicchetti, G.; Calandriello, L.; Del Ciello, A.; Bonomo, L. Lung nodules: Size still matters. Eur. Respir. Rev. 2017, 26, 170025. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, A.; Michelini, S.; Nocivelli, G.; Silva, M.; Scrimieri, A.; Pezzotti, S.; Maroldi, R.; Farina, D. Solid Indeterminate Pulmonary Nodules Less Than or Equal to 250 mm3: Application of the Updated Fleischner Society Guidelines in Clinical Practice. Radiol. Res. Pract. 2019, 2019, 7218258. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, A.; Michelini, S.; Scrimieri, A.; Golemi, S.; Maroldi, R. Solid Indeterminate Pulmonary Nodules of Less Than 300 mm3: Application of Different Volume Doubling Time Cut-offs in Clinical Practice. Diagnostics 2019, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, Y.; Ambrogio, C.; Mitsudomi, T. Ground-glass nodules of the lung in never-smokers and smokers: clinical and genetic insights. Transl. Lung Cancer Res. 2018, 7, 487–497. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Mitsudomi, T. Management of ground-glass opacities: should all pulmonary lesions with ground-glass opacity be surgically resected? Transl. Lung Cancer Res. 2013, 2, 354–363. [Google Scholar]
- Borghesi, A.; Farina, D.; Michelini, S.; Ferrari, M.; Benetti, D.; Fisogni, S.; Tironi, A.; Maroldi, R. Pulmonary adenocarcinomas presenting as ground-glass opacities on multidetector CT: Three-dimensional computer-assisted analysis of growth pattern and doubling time. Diagn. Interv. Radiol. 2016, 22, 525–533. [Google Scholar] [CrossRef]
- Cohen, J.G.; Reymond, E.; Lederlin, M.; Medici, M.; Lantuejoul, S.; Laurent, F.; Arbib, F.; Jankowski, A.; Moreau-Gaudry, A.; Ferretti, G.R. Differentiating pre- and minimally invasive from invasive adenocarcinoma using CT-features in persistent pulmonary part solid nodules in Caucasian patients. Eur. J. Radiol. 2015, 84, 738–744. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, C.M.; Lee, S.M.; Kim, H.; McAdams, H.P.; Goo, J.M. Persistent pulmonary subsolid nodules with solid portions of 5 mm or smaller: Their natural course and predictors of interval growth. Eur. Radiol. 2016, 26, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yankelevitz, D.F.; Kostakoglu, L.; Beasley, M.B.; Htwe, Y.; Salvatore, M.M.; Yip, R.; Henschke, C.I. Updating the role of FDG PET/CT for evaluation of lung cancer manifesting in nonsolid nodules. Clin. Imaging 2018, 52, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kazerooni, E.A.; Austin, J.H.M.; Black, W.C.; Dyer, D.S.; Hazelton, T.R.; Leung, A.N.; McNitt-Gray, M.F.; Munden, R.F.; Pipavath, S. ACR-STR practice parameter for the performance and reporting of lung cancer screening thoracic computed tomography (CT): 2014 (Resolution 4). J. Thorac. Imaging 2014, 29, 310–316. [Google Scholar] [CrossRef]
- Song, Y.S.; Park, C.M.; Park, S.J.; Lee, S.M.; Jeon, Y.K.; Goo, J.M. Volume and mass doubling times of persistent pulmonary subsolid nodules detected in patients without known malignancy. Radiology 2014, 273, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, C.M.; Kim, H.; Hwang, E.J.; Park, J.; Goo, J.M. Persistent part-solid nodules with solid part of 5 mm or smaller: Can the ‘follow-up and surgical resection after interval growth’ policy have a negative effect on patient prognosis? Eur. Radiol. 2017, 27, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R.S. Evaluation of Individuals with Pulmonary Nodules: When is it Lung Cancer? Diagnosis and Management of Lung Cancer, 3rd ed.; American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e93S–e120S. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Shimizu, Y.; Yamamoto, T.; Yoshikawa, J.; Hashizume, Y. Predictive value of one-dimensional mean computed tomography value of ground-glass opacity on high-resolution images for the possibility of future change. J. Thorac. Oncol. 2014, 9, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Kondo, R.; Kawakami, S.; Matsushita, M.; Yoshizawa, A.; Hara, D.; Matsuoka, S.; Takeda, T.; Miura, K.; Agatsuma, H.; et al. Computed tomography attenuation predicts the growth of pure ground-glass nodules. Lung Cancer 2014, 84, 242–247. [Google Scholar] [CrossRef]
- Bak, S.H.; Lee, H.Y.; Kim, J.H.; Um, S.W.; Kwon, O.J.; Han, J.; Kim, H.K.; Kim, J.; Lee, K.S. Quantitative CT Scanning Analysis of Pure Ground-Glass Opacity Nodules Predicts Further CT Scanning Change. Chest 2016, 149, 180–191. [Google Scholar] [CrossRef]
- Borghesi, A.; Michelini, S.; Bertagna, F.; Scrimieri, A.; Pezzotti, S.; Maroldi, R. Hilly or mountainous surface: A new CT feature to predict the behavior of pure ground glass nodules? Eur. J. Radiol. Open 2018, 5, 177–182. [Google Scholar] [CrossRef]
- Sun, Q.; Huang, Y.; Wang, J.; Zhao, S.; Zhang, L.; Tang, W.; Wu, N. Applying CT texture analysis to determine the prognostic value of subsolid nodules detected during low-dose CT screening. Clin. Radiol. 2019, 74, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Deng, J.; She, Y.; Zhang, L.; Ren, Y.; Sun, W.; Su, H.; Dai, C.; Jiang, G.; Sun, X.; et al. Quantitative features can predict further growth of persistent pure ground-glass nodule. Quant. Imaging Med. Surg. 2019, 9, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 11 March 2019).
- Olson, E. Particle shape factors and their use in image analysis-Part 1: Theory. J. GXP Compliance 2011, 15, 85–95. [Google Scholar]
- ISO. ISO 9276-6:2008(E)—Representation of Results of Particle Size Analysis–Part 6: Descriptive and Quantitative Representation of Particle Shape and Morphology; ISO: Geneve, Switzerland, 2008; pp. 1–23. [Google Scholar]
- Mull, R.T. Mass estimates by computed tomography: Physical density from CT numbers. AJR Am. J. Roentgenol. 1984, 143, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- de Hoop, B.; Gietema, H.; van de Vorst, S.; Murphy, K.; van Klaveren, R.J.; Prokop, M. Pulmonary ground-glass nodules: Increase in mass as an early indicator of growth. Radiology 2010, 255, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Ko, J.P.; Berman, E.J.; Kaur, M.; Babb, J.S.; Bomsztyk, E.; Greenberg, A.K.; Naidich, D.P.; Rusinek, H. Pulmonary Nodules: Growth rate assessment in patients by using serial CT and three-dimensional volumetry. Radiology 2012, 262, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Tantucci, C.; Bottone, D.; Borghesi, A.; Guerini, M.; Quadri, F.; Pini, L. Methods for Measuring Lung Volumes: Is There a Better One? Respiration 2016, 91, 273–280. [Google Scholar] [CrossRef]
- Silva, M.; Milanese, G.; Seletti, V.; Ariani, A.; Sverzellati, N. Pulmonary quantitative CT imaging in focal and diffuse disease: Current research and clinical applications. Br. J. Radiol. 2018, 91, 20170644. [Google Scholar] [CrossRef]
- Ravanelli, M.; Agazzi, G.M.; Ganeshan, B.; Roca, E.; Tononcelli, E.; Bettoni, V.; Caprioli, A.; Borghesi, A.; Berruti, A.; Maroldi, R.; et al. CT texture analysis as predictive factor in metastatic lung adenocarcinoma treated with tyrosine kinase inhibitors (TKIs). Eur. J. Radiol. 2018, 109, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Gawlitza, J.; Sturm, T.; Spohrer, K.; Henzler, T.; Akin, I.; Schönberg, S.; Borggrefe, M.; Haubenreisser, H.; Trinkmann, F. Predicting Pulmonary Function Testing from Quantified Computed Tomography Using Machine Learning Algorithms in Patients with COPD. Diagnostics 2019, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, A.; van Ginneken, B.; Nair, A.; Baldwin, D. Use of Volumetry for Lung Nodule Management: Theory and Practice. Radiology 2017, 284, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Goo, J.M. A computer-aided diagnosis for evaluating lung nodules on chest CT: The current status and perspective. Korean J. Radiol. 2011, 12, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fan, L.; Cao, E.T.; Li, Q.C.; Gu, Y.F.; Liu, S.Y. Quantitative CT analysis of pulmonary pure ground-glass nodule predicts histological invasiveness. Eur. J. Radiol. 2017, 89, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Nemec, U.; Heidinger, B.H.; Anderson, K.R.; Westmore, M.S.; VanderLaan, P.A.; Bankier, A.A. Software-based risk stratification of pulmonary adenocarcinomas manifesting as pure ground glass nodules on computed tomography. Eur. Radiol. 2018, 28, 235–242. [Google Scholar] [CrossRef]
- Han, L.; Zhang, P.; Wang, Y.; Gao, Z.; Wang, H.; Li, X.; Ye, Z. CT quantitative parameters to predict the invasiveness of lung pure ground-glass nodules (pGGNs). Clin. Radiol. 2018, 73, 504.e1–504.e7. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Heuvelmans, M.A.; Zhang, H.; Oudkerk, M.; Zhang, G.X.; Xie, X.Q. Changes in quantitative CT image features of ground-glass nodules in differentiating invasive pulmonary adenocarcinoma from benign and in situ lesions: histopathological comparisons. Clin. Radiol. 2018, 73, 504.e9–504.e16. [Google Scholar] [CrossRef]
- Fan, L.; Fang, M.; Li, Z.; Tu, W.; Wang, S.; Chen, W.; Tian, J.; Dong, D.; Liu, S. Radiomics signature: A biomarker for the preoperative discrimination of lung invasive adenocarcinoma manifesting as a ground-glass nodule. Eur. Radiol. 2019, 29, 889–897. [Google Scholar] [CrossRef]
- Kim, H.Y.; Shim, Y.M.; Lee, K.S.; Han, J.; Yi, C.A.; Kim, Y.K. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology 2007, 245, 267–275. [Google Scholar] [CrossRef]
- Albano, D.; Borghesi, A.; Bosio, G.; Bertoli, M.; Maroldi, R.; Giubbini, R.; Bertagna, F. Pulmonary mucosa-associated lymphoid tissue lymphoma: (18) F-FDG PET/CT and CT findings in 28 patients. Br. J. Radiol. 2017, 90, 20170311. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Kim, M.A.; Park, C.M.; Lee, C.H.; Goo, J.M.; Lee, H.J. Ground-glass nodules found in two patients with malignant melanomas: Different growth rate and different histology. Clin. Imaging 2010, 34, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, C.M.; Koh, J.M.; Lee, S.M.; Goo, J.M. Pulmonary subsolid nodules: What radiologists need to know about the imaging features and management strategy. Diagn. Interv. Radiol. 2014, 20, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Kadakia, S.; Ambur, V.; Muro, K.; Kaiser, L. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J. Thorac. Cardiovasc. Surg. 2017, 153, 1581–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milanese, G.; Sverzellati, N.; Pastorino, U.; Silva, M. Adenocarcinoma in pure ground glass nodules: Histological evidence of invasion and open debate on optimal management. J. Thorac. Dis. 2017, 9, 2862–2867. [Google Scholar] [CrossRef] [PubMed]






| Characteristic | |
|---|---|
| Age (years) | 65.5 ± 10.5 |
| Sex | |
| Male | 24 (48.0) |
| Female | 26 (52.0) |
| Smoking habits | |
| Current/former | 35 (70.0) |
| Never | 15 (30.0) |
| Oncologic history | 23 (46.0) |
| Non-small cell lung cancer | 10 (43.5) |
| Breast cancer | 4 (17.4) |
| Head and neck cancer | 3 (13.0) |
| Other malignancy | 6 (26.1) |
| CT Feature | Value | Spearman’s Rho * | p Value * |
|---|---|---|---|
| Area (mm2) | 67 (40.5–86.8) | −0.306 | 0.031 |
| Perimeter (mm) | 36.9 (27.6–46.4) | −0.449 | 0.001 |
| Mean Feret diameter (mm) | 11 (8.3–13.2) | −0.365 | 0.009 |
| Mean attenuation (HU) | −497.3 (−563.5–−449) | −0.069 | 0.633 |
| Median attenuation (HU) | −534.5 (−605–−478) | −0.019 | 0.897 |
| Modal attenuation (HU) | −703 (−758–−596) | −0.029 | 0.842 |
| Standard deviation (HU) | 226 (202.2–248.6) | −0.241 | 0.092 |
| Skewness | 0.67 (0.46–0.91) | −0.003 | 0.983 |
| Kurtosis | −0.17 (−0.61–0.53) | 0.124 | 0.390 |
| LMD (mg/mm) | 31.4 (20–43.5) | −0.326 | 0.021 |
| Circularity | 0.64 (0.52–0.75) | 0.519 | <0.001 |
| Solidity | 0.81 (0.74–0.87) | 0.457 | <0.001 |
| CT Feature | Growing PSNs | Nongrowing PSNs | p Value * |
|---|---|---|---|
| Area (mm2) | 76.7 (45.1–96.1) | 45.6 (36.6–66.9) | 0.008 |
| Perimeter (mm) | 42.5 (34.3–49.6) | 30 (24.2–36.7) | <0.001 |
| Mean Feret diameter (mm) | 12.6 (9.4–13.7) | 8.6 (7.6–10.7) | 0.002 |
| Mean attenuation (HU) | −490.5 (−551–−442.6) | −504.5 (−585.3–−455.6) | 0.362 |
| Median attenuation (HU) | −521 (−594.5–−476) | −548 (−618–−499) | 0.566 |
| Modal attenuation (HU) | −716 (−756.5–−647.5) | −674 (−765.0–−574) | 0.401 |
| Standard deviation (HU) | 233 (204.4–255.9) | 204.8 (188.8–225.3) | 0.013 |
| Skewness | 0.7 (0.47–0.93) | 0.67 (0.33–0.90) | 0.846 |
| Kurtosis | −0.33 (−0.59–0.29) | 0.16 (−0.63–0.63) | 0.676 |
| LMD (mg/mm) | 35.3 (22.8–44.2) | 21.6 (18.1–30) | 0.007 |
| Circularity | 0.59 (0.46–0.66) | 0.76 (0.64–0.82) | <0.001 |
| Solidity | 0.78 (0.71–0.84) | 0.88 (0.82–0.93) | 0.001 |
| Independent Variables | PSN Outcome | χ2 Test | |
|---|---|---|---|
| Growing (n = 33) | Nongrowing (n = 17) | p Value | |
| Patient age | 0.276 | ||
| ≤65 years | 14 (28) | 10 (20) | |
| >65 years | 19 (38) | 7 (14) | |
| Patient sex | 0.062 | ||
| Male | 19 (38) | 5 (10) | |
| Female | 14 (28) | 12 (24) | |
| Smoking history | 0.061 | ||
| No | 7 (14) | 8 (16) | |
| Yes | 26 (52) | 9 (18) | |
| Oncologic history | 0.004 | ||
| No | 13 (26) | 14 (28) | |
| Yes | 20 (40) | 3 (6) | |
| Emphysema | 0.562 | ||
| No | 24 (48) | 11 (22) | |
| Yes | 9 (18) | 6 (12) | |
| Lobe location | 0.247 | ||
| Middle/lower | 10 (20) | 8 (16) | |
| Upper | 23 (46) | 9 (18) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borghesi, A.; Scrimieri, A.; Michelini, S.; Calandra, G.; Golemi, S.; Tironi, A.; Maroldi, R. Quantitative CT Analysis for Predicting the Behavior of Part-Solid Nodules with Solid Components Less than 6 mm: Size, Density and Shape Descriptors. Appl. Sci. 2019, 9, 3428. https://doi.org/10.3390/app9163428
Borghesi A, Scrimieri A, Michelini S, Calandra G, Golemi S, Tironi A, Maroldi R. Quantitative CT Analysis for Predicting the Behavior of Part-Solid Nodules with Solid Components Less than 6 mm: Size, Density and Shape Descriptors. Applied Sciences. 2019; 9(16):3428. https://doi.org/10.3390/app9163428
Chicago/Turabian StyleBorghesi, Andrea, Alessandra Scrimieri, Silvia Michelini, Giulio Calandra, Salvatore Golemi, Andrea Tironi, and Roberto Maroldi. 2019. "Quantitative CT Analysis for Predicting the Behavior of Part-Solid Nodules with Solid Components Less than 6 mm: Size, Density and Shape Descriptors" Applied Sciences 9, no. 16: 3428. https://doi.org/10.3390/app9163428