1. Introduction
Photosynthetic bacteria, which are common microorganisms in the natural environment, have been applied in the field of environmental protection, such as in the treatment of sewage, domestic wastewater and the bioremediation of sediment mud polluted with organic matter (see, for example, [
1,
2,
3,
4]). On the other hand, photosynthetic bacteria can produce relatively large amounts of physiologically-active substances, such as vitamin
, ubiquinone (coenzyme
), 5-aminolevulinic acid (ALA) and RNA (see, for example [
5]). In particular, vitamin
has been used in treating anemia and as an eye lotion. Recently, applications as health food supplements have received considerable attention. Coenzyme
has been used in treating heart diseases for many years. Further, coenzyme
has been used not only as a medicine, but also as some food supplements, because of its physiological activities. One of the developments of ALA applications is in the area of photodynamic diagnosis. RNA is an attractive source of 5
-ribonucleotides for use as a flavor enhancer in the food industry. In recent years, the production of RNA has been used as a dietary source of pyrimidine for human immune functions (see, for example [
6,
7]).
Some photosynthetic bacteria, such as
, are extensively used in the production of lycopene, aquaculture, and so on [
8]. It can use sunlight, inorganic and organic compounds for energy. Further,
can have practical value for removing microcystin from the water body during algal blooms [
9]. It can also degrade 2,4,6-trinitrotoluene (TNT), which has negative effects on the human body and aquatic life, resulting in a major threat to drinking and irrigation water supplies, as well as the recreational use of surface waters worldwide. Moreover,
is regarded as the most promising microbial system for the biological production of hydrogen, which has been extensively developed because of its high-energy content and clean product after combustion [
10]. However, the concentration of
is very low under anaerobic light culture conditions (see, for example, [
11,
12]). Therefore, cost-efficient harvesting of
is a new challenge. In order to harvest
from the liquid, it is necessary to flocculate the single cells into large cell aggregates. Flocculation is a chemically-based separation process that requires less energy than centrifugation and ultrafiltration and, thus, is regarded as the most promising means for degrading microorganisms. Since algal toxins of blooms have happened occasionally in recent years, the problems of degrading microorganisms have received wide attentions (see, for example, [
13,
14,
15,
16]).
Flocculants are a kind of important water treatment reagent, which can be divided into organic flocculants and inorganic flocculants according to the chemical compositions [
17]. Although organic flocculants, such as polyacrylamide, are frequently used in wastewater treatment and industrial downstream processes because of their high efficiency, some of them are not easily degraded in nature [
18,
19], and some of the monomers derived from synthetic polymers are harmful to the human body (see, for example, [
20,
21]). To solve these environmental problems, inorganic flocculants are increasingly being seen as an alternative in the settlement of microorganisms, more specifically in wastewater treatment owing to their inexpensive and nontoxic characteristics. Thus, inorganic flocculants may be used as nontoxic, cost-effective and widely-available flocculants for harvesting
(see, for example, [
22,
23,
24,
25,
26,
27] and the references therein).
Mathematical models have played an important role in better understanding microbiology and population biology (see, for example, [
28,
29]). In recent years, the dynamics of the chemostat models has received considerable attentions (see, for example, [
29]). The article of Smith and Waltman has played an important role in the development of the chemostat models [
30]. From then on, much research on the chemostat models has been extensively studied by many authors. A model describing two populations of microorganisms competing for one single limiting nutrients was proposed in [
31]. Later, the model was extended to an arbitrary number of populations in [
32,
33]. These models, which were studied in articles [
31,
32,
33], have proven that they all include a competitive exclusion effect. In the articles [
34,
35,
36,
37,
38,
39], some further developments have been performed on the chemostat models to place the relevant models in a naturally more sensible manner.
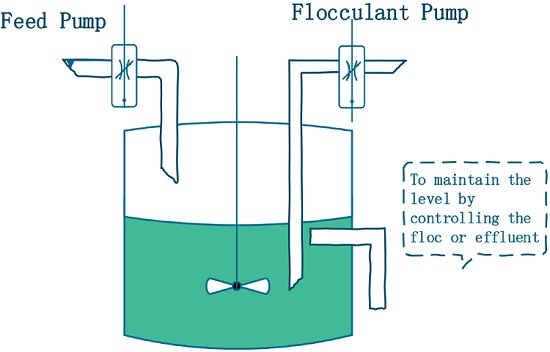
Let
and
denote the concentration of the nutrients and
, respectively, in the culture vessel at time
t.
denotes the concentration of inorganic flocculants, which are used for harvesting
(see
Figure 1). The constants
and
denote the input concentration of the nutrients and flocculants, respectively. For simplicity, it is assumed that the input of the nutrients and flocculants is continuous. The constant
is the dilution rate of the chemostat.
The constant
denotes the time delay involved in the conversion of nutrients to
. The flocculation rate of microorganisms is assumed to be a bilinear mass-action function response
, where
is the per capita contact rate. At the same time, flocculants produce loss or consumption [
17], and the loss rate of flocculants is also assumed to be a bilinear mass-action function response
, where
is constant. Thus, in [
40], the following dynamic model has been proposed:
In Model (1), and are constants, and the bilinear mass-action uptake function has been used.
It should be mentioned here that, the analysis reveals that Model (1) proposed in [
40] exhibits the phenomenon of backward bifurcation for the existence of positive equilibria. Moreover, the local stability properties of the equilibria have been dealt with in detail.
People found that the influence of different nutrients has played an important role in the culture of microorganisms. In order to take this into consideration, appropriate combinations of nutrients are considered in chemostat models. Models with two competitors and two perfectly-complemented growth-limiting nutrients are studied in [
41,
42]. Local asymptotic conditions for the equilibria are derived.
When there is a microorganism to compete for two or more resources, it may become necessary to consider how the resources, once consumed, interact to promote growth. In [
41], the authors employ consumer needs to provide a criterion to classify resources and classify resources as perfectly complementary, perfectly substitutable or imperfectly substitutable. Perfectly-complementary resources are different essential substances that must be taken together. In this case, each substance fulfills different functions with respect to the growth of microorganisms. For example, carbon source and nitrogen source may be complementary for the growth of bacterium.
Motivated by the papers mentioned above, in this paper, we further consider a dynamic model describing the cultivation and flocculation of
, and the nutrients presented in [
40] will be divided into carbon source and nitrogen source, which are perfectly complementary in the culture of
(see, for example, [
5,
41,
42,
43,
44]). We assume that the growth of
is always co-limited by carbon and nitrogen for all possible nutrient conditions. We do not consider the case where only one of these nutrients limits growth, for example, in environmental scenarios of high carbon, but very low nitrogen loads, the growth of bacteria may be purely nitrogen limited.
Let
and
denote the concentration of carbon source and nitrogen source, respectively, in the culture vessel at time
t. The constants
and
denote the input concentration of carbon source and nitrogen source, respectively. We assume that the conversion of nutrients to microorganism biomass occurs instantly. That is, the time delay
τ in Model (1) equals zero. Hence, we have the following dynamic model describing the cultivation and flocculation of a microorganism:
In Model (2), the parameters
D,
r,
,
and
are the same as Model (1). The term
is the growth rate of
, and the terms
and
represent the quantity of the decreasing of the carbon source and nitrogen source, respectively, where
and
are non-negative constants; the functions
and
are nonnegative and continuous for
,
. For the simplicity of the theoretical analysis, in this paper, the functions
and
are chosen as Monod-type functions, i.e.,
where
and
are the half-saturation constants with respect to the carbon source and nitrogen source, respectively.
are yield coefficients (see, for example, [
29]), which are defined as:
Therefore, the dynamic Model (2) can be rewritten in the following form:
It is convenient to introduce dimensionless variables. In particular, we define:
and still denote
,
,
,
,
,
,
,
and
with
,
,
,
t,
,
,
r,
and
, then Model (3) becomes:
According to the biological considerations, the initial condition of Model (4) is given as:
where the constants
and
represent the initial concentration of the carbon source, nitrogen source,
and flocculants respectively.
The purpose of this paper is to tackle the existence of backward and forward bifurcations by using center manifold theory and to investigate the global stability properties of the two classes of equilibria by constructing the suitable Lyapunov functions.
The organization of the paper is as follows. The global existence, nonnegativity and boundedness of the solutions of Model (4) are investigated in
Section 2. In
Section 3, the existence of the equilibria and the phenomena of backward and forward bifurcations are extensively discussed. In
Section 4, the global stability of the boundary equilibrium of Model (4) is discussed by the stability theory of ordinary differential equations. Furthermore, we consider the local stability of positive equilibrium, the uniform persistence of Model (4) and the global asymptotic stability of the positive equilibrium in
Section 5. In
Section 6, some control strategies are given by the theoretical analysis. Some discussions are given in
Section 7.
2. The Global Existence, Nonnegativity and Boundedness of Solutions
From the biological considerations, it is necessary to show that all of the solutions of Model (4) are nonnegative and bounded for all
. By using the basic theory of ordinary differential equations [
45] and some simple calculations, it is not difficult to show the following result.
Theorem 1. The solution of Model (4) with the initial Condition (5) is existent, unique and nonnegative for all and satisfies:where ,
,
=
=
Proof. From the theory of the local existence of solutions for ordinary differential equations, it can be obtained that the solution
of Model (4) is existent and unique for
. Here,
δ is some positive constant [
29,
45,
46]. Furthermore, we also have that the solution
is nonnegative for
.
We can easily show that
,
and
are bounded on
. Let us further show that
is also bounded on
. For
, define:
From Model (4), we obtain that, for
,
From (6) and the well-known comparison principle, we have that
is also bounded for
. Hence, by employing the continuation theorems of the solutions [
29,
45,
46], the solution
is existent and unique for any
. Similarly, the solution is nonnegative for any
.
Thus, from the comparison principle, we have that:
By the first equation of Model (4), we obtain that, for
,
Again, we can conclude that By using the technique similar above, we can show that We complete the proof of Theorem 1. □
Theorem 2. The compact set:attracts all of the solutions of Model (4) and is positively invariant with respect to Model (4). Proof. According to Theorem 1, it only needs to be proven that Ω is positively invariant with respect to Model (4). That is, it needs to be shown that for any if . Let us show for any .
In fact, if there exists some , such that , then is existent and . Hence, we obtain that , and for . By the Lagrange mean-value theorem, there exists some , such that
On the other hand, from (6), we have that which is a contradiction. Thus, for any . Therefore, from Model (4), we have that for any , , from which we easily have that , , for any .
Next, we prove that for any if , , , .
In fact, if there exists some , such that , then is existent and . Hence, we have that , and for . By the Lagrange mean-value theorem, there exists some , such that
On the other hand, from Model (4), we obtain that:
which is a contradiction. Thus,
for any
. Therefore, from Model (4), we obtain that, for any
,
from which we easily obtain that
,
, for any
. This completes the proof of Theorem 2. □
3. The Existence of the Equilibria and Its Classification
Let
be any equilibrium of Model (4). Then,
satisfies the following nonlinear algebraic equations,
Model (4) always has the boundary equilibrium . The existence of indicates that, if there is no to be added into the culture vessel at the beginning of the culture, the concentrations of the carbon source, nitrogen source and flocculants always maintain the constant values 1, 1 and 1, respectively. The equilibrium is also called -free equilibrium.
Define the basic bifurcation parameter as:
Let
be any positive equilibrium of Model (4). From (7), we have that:
Clearly,
should satisfy the following conditions:
Substituting the second, third and forth equations of (8) into the first equation gives a fifth order algebraic equation,
where:
Let us consider the necessary condition for the existence of the positive equilibria of Model (4).
From the first equation in (8), we obtain the following function:
which implies that
is a necessary condition for a positive equilibrium to exist.
Using the methods similar to [
47], we can give the sufficient conditions of the existence of the positive equilibria of Model (4). The following results (Theorem 3) follow from the various possibilities enumerated in
Table A1 (see
Appendix A):
Theorem 3. - (i)
Model (4) has a unique positive equilibrium if and Conditions (9) hold and whenever Cases 1, 9, 13, 15 and 16 in Table A1 are satisfied; - (ii)
Model (4) could have more than one positive equilibrium if and Conditions (9) hold and whenever Cases 2–8, 10–12 and 14 in Table A1 are satisfied; - (iii)
Model (4) could have five positive equilibria at most if and Conditions (9) hold and whenever Cases 1–16 in Table A1 are satisfied.
Hence, under suitable conditions, there may be at most five different positive roots for the fifth order algebraic equation. Let be any such positive root, which also satisfies Conditions . Thus, from (8), , and can be obtained. Therefore, Model (4) at most has five positive equilibria of the type of . The equilibrium indicates that the carbon source, nitrogen source, and flocculants may be coexistent for any time .
Remark 1. The existence of multiple positive equilibria of Model (4) when (shown in Table A1; see Appendix A) indicates the possibility of the existence of backward bifurcation (see, for example, [40,47,48]), where the stable boundary equilibrium co-exists with a stable positive equilibrium. This can be explored below via numerical simulations (see Figure 2a,b). A rigorous result can be obtained using center manifold theory [49]. The detailed proof is given in Appendix B. From the analysis given in
Appendix B, we have established the following result.
Theorem 4. - (i)
If:then Model (4) undergoes a backward bifurcation at .
- (ii)
If:then Model (4) undergoes a forward bifurcation at .
The existence of backward bifurcation implies that stable boundary equilibrium and stable positive equilibrium may be coexistent. In biology, this means that the basic bifurcation parameter is not the threshold value, which is used to determine whether can be harvested successfully or not. In this case, more complicated dynamic properties may occur. There may exist a new threshold value, which is less than and used to determine whether can be harvested successfully or not.
4. The Global Stability of the Boundary Equilibrium
Global stability properties of the equilibria or imply that the asymptotic properties of the carbon source, nitrogen source, and flocculants in the culture vessel are not dependent on the initial values and . For the global stability property of the boundary equilibrium of Model (4), we have the following result.
Theorem 5. If , then the boundary equilibrium of Model (4) is locally asymptotically stable. Further, if:then the boundary equilibrium of Model (4) is globally asymptotically stable. Proof. It is easy to show that the boundary equilibrium of Model (4) is locally asymptotically stable by the characteristic equation of the linearization of Model (4). Next, we prove the global asymptotic stability of the boundary equilibrium of Model (4).
Since Ω is attractive and positively forward invariant for Model (4), hence it just considers Model (4) in Ω. Define:
Apparently,
and
is continuous on Ω. If
, then the derivative of
along the solutions of Model (4) is:
for any
. Hence,
is a Lyapunov function of Model (4) on Ω.
Define
We have that:
Let be the largest set in E, which is invariant with respect to Model (4). Clearly, is not empty, since For any , let be the solution of Model (4) with the initial Condition (5). From the invariance of , we get for any . Thus, we get, for each t, , or , and .
If for some , and , then we have that . Hence, from the first or second equation of Model (4), we obtain that . Thus, for any , we have that .
Subsequently, from the first, second and forth equations of Model (4), we have that, for any
,
Furthermore, for any
, we have that:
If or or , then , and become negative values . Then, we obtain that , and . Hence, we obtain that, for any , Therefore, we obtain The classical Lyapunov–LaSalle invariance principle shows that is globally attractive. Since it has been shown that, if , the boundary equilibrium of Model (4) is locally asymptotically stable. Hence, the boundary equilibrium of Model (4) is globally asymptotically stable. □
Remark 2. Let us use some numerical simulations to check the correctness of the theoretical analyses. We setBy the analysis of Theorem 5, we obtain that the boundary equilibrium of Model (4) is globally asymptotically stable (see Figure 3a). Furthermore, if the parameters are chosen as follows, it is easy to check that the condition holds, but there are two positive equilibria, and Then, the backward bifurcation phenomenon is illustrated. Therefore, sufficient Condition (10) is reasonable.
6. Control Strategies
In this section, some control strategies are provided by suitable theoretical analysis. If
holds, then, we have from Theorem 5 that the boundary equilibrium
of Model (4) is locally asymptotically stable. We note that the condition
is equivalent to the following inequality,
All of the parameters
r,
,
and
in (12) are defined as in Model (4). (12) can be further written as the following form,
Here, all of the parameters r, , , , , , and D in (13) are defined as in Model (3).
In view of the biological meanings of the parameters in Model (3) and Condition , Theorem 5 indicates that the concentration of in the chemostat tends to zero, and the concentration of the carbon source, nitrogen source and flocculants may tend to the constant values , and , respectively, as time t increases, if one of the following two cases occurs: (a) reducing the absorption of or the carbon input concentration, or the nitrogen input concentration; (b) improving the velocity or the flocculation effect or flocculant input concentration. These cases are reasonable, since they imply the insufficient sources for to grow. Hence, in the environmental science field, it can be used to remove algae and heavy metals.
From Theorem 7, we have that Model (3) is uniformly persistent if . This means that the concentrations of the carbon source, nitrogen source, and flocculants in the chemostat may be ultimately maintained at some positive constant values, as time t increases, if one of the following two cases occurs: (a) improving the absorption of , or the carbon input concentration, or the nitrogen input concentration; (b) reducing the velocity or the flocculation effect or flocculant input concentration.
These control strategies can be performed by numerical simulations.
In the following, for convenience, we simulate the extinction or persistence of microorganism () numerically by using (12) and Model (4).
If the parameters are chosen as in
Table A2 (see
Appendix A),
in the chemostat will tend to extinction (see
Figure 4a).
If the absorption of
(
r) is improved from
to
and the other parameters are the same as
Table A2,
in the chemostat will tend to be constant (see
Figure 4b).
If the flocculation effect (
) is reduced from
to
,
in the chemostat will tend to be constant (see
Figure 5a).
If Michaelis–Menten constant of carbon (
) is reduced from
to
,
in the chemostat will tend to be constant (see
Figure 5b).
If Michaelis–Menten constant of nitrogen (
) is reduced from
to
,
in the chemostat will tend to be constant (see
Figure 6).
7. Discussion and Conclusions
In the paper, based on some biological considerations and chemostat models, a dynamic model governed by ordinary differential equations with four variables (carbon source, nitrogen source, and flocculants) is presented. There is a boundary equilibrium and at most five positive equilibria for the proposed model. To give a theoretical analysis for the existence of all of the positive equilibria of Model (4), the method of the Descartes rule of signs is applied to the classifications of the positive roots of a fifth order algebraic equation.
The local and global stability properties of the boundary equilibrium of Model (4) have been studied in detail. An interesting phenomenon of backward and forward bifurcations is observed. That is, there may exist two positive equilibria even if the condition holds. Hence, sufficient Condition (10) to ensure the global stability of the boundary equilibrium is reasonable in mathematics.
The local stability of the positive equilibrium of Model (4) is also carried out. From Condition (11), we have that the positive equilibrium is locally asymptotically stable when the flocculation coefficient is small enough. Hence, Condition (11) is also reasonable in biology.
Uniform persistence of Model (4) has also been completely studied under the condition
. Uniform persistence has very important significance both in mathematics and biology, and it characterizes the long-term survival of some microorganisms [
45].
Finally, some control strategies are provided by simple theoretical analysis. From Theorem 5, we have that in the chemostat will tend to extinction if . In this case, these control strategies can be applied to remove , which are well known to produce a variety of toxins and have serious harm on human health. From Theorem 7, we have that in the chemostat will tend to be positive constant if . In this case, these control strategies can be widely used for the collection of useful microorganisms.
It is well-known that the existence of time delays is inevitable in biology. For example, in the cultivation of microorganisms, there are always time delays in the process of transferring nutrients and the uptake of nutrients. Hence, chemostat models with time delays that account for the time lapsing between the uptake of nutrients by cells and the incorporation of these nutrients as biomass have been given much attention [
40,
55,
56,
57]. Based on Model (3), it may have the following more general form with time delays,
In Model (14), the constants and are the rate constants at which the carbon source and nitrogen source are recycled because of the death of . The constant is a fixed time during which the carbon source and nitrogen source are released completely from dead . The constant denotes the time delay involved in the conversion of nutrients to . The factor is the probability constant at which remains in the culture vessel during the conversion process. The theoretical analysis of Model (14) will be studied separately.