Effects of Anaerobic Digestates and Biochar Amendments on Soil Health, Greenhouse Gas Emissions, and Microbial Communities: A Mesocosm Study
Abstract
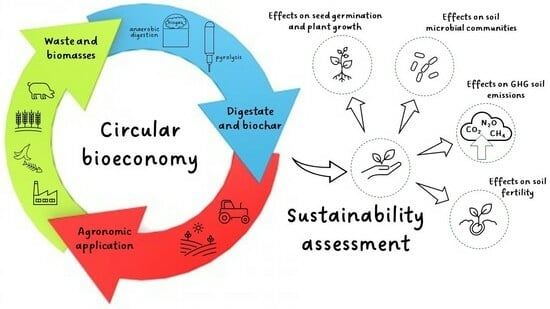
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. External Organic Materials
2.2. Germination Index
2.3. Soil Collection and Characteristics
2.4. Mesocosm Experiment Setup
2.5. GHG Emissions and Soil Analysis
2.6. DNA Extraction and Microbial Community Structure
2.7. Statistical Analysis
3. Results
3.1. Seed Germination Index
3.2. Changes in Soil Properties
3.3. GHG Emissions
3.4. Microbial Community Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gontard, N.; Sonesson, U.; Birkved, M.; Majone, M.; Bolzonella, D.; Celli, A.; Angellier-Coussya, H.; Jangg, G.W.; Verniqueth, A.; Broezei, J.; et al. A research challenge vision regarding management of agricultural waste in a circular bio-based economy. Crit. Rev. Environ. Sci. Technol. 2018, 48, 614–654. [Google Scholar] [CrossRef]
- Liu, H.; Kumar, V.; Yadav, V.; Guo, S.; Sarsaiya, S.; Binod, P.; Sindhu, R.; Xu, P.; Zhang, Z.; Pandey, A.; et al. Bioengineered biochar as smart candidate for resource recovery toward circular bio-economy: A review. Bioengineered 2021, 12, 10269–10301. [Google Scholar] [CrossRef]
- Singh, R.; Paritosh, K.; Pareek, N.; Vivekanand, V. Integrated system of anaerobic digestion and pyrolysis for valorization of agricultural and food waste towards circular bioeconomy. Bioresour. Technol. 2022, 360, 127596. [Google Scholar] [CrossRef]
- Vautrin, F.; Piveteau, P.; Cannavacciuolo, M.; Barré, P.; Chauvin, C.; Villenave, C.; Cluzeau, D.; Hoeffner, K.; Mulliez, P.; Jean-Baptiste, V.; et al. The short-term response of soil microbial communities to digestate application depends on the characteristics of the digestate and soil type. Appl. Soil Ecol. 2024, 193, 105105. [Google Scholar] [CrossRef]
- Mickan, B.S.; Ren, A.T.; Buhlmann, C.H.; Ghadouani, A.; Solaiman, Z.M.; Jenkins, S.; Pang, J.; Ryan, M.H. Closing the circle for urban food waste anaerobic digestion: The use of digestate and biochar on plant growth in potting soil. J. Clean. Prod. 2022, 347, 131071. [Google Scholar] [CrossRef]
- Da Ros, C.; Libralato, G.; Ghirardini, A.V.; Radaelli, M.; Cavinato, C. Assessing the potential phytotoxicity of digestate from winery wastes. Ecotoxicol. Environ. Saf. 2018, 150, 26–33. [Google Scholar] [CrossRef]
- Suproniene, S.; Doyeni, M.O.; Viti, C.; Tilvikiene, V.; Pini, F. Characterization of the Soil Prokaryotic community with respect to time and fertilization with animal waste–based digestate in a humid continental climate. Front. Environ. Sci. 2022, 10, 852241. [Google Scholar] [CrossRef]
- Ren, A.T.; Abbott, L.K.; Chen, Y.; Xiong, Y.C.; Mickan, B.S. Nutrient recovery from anaerobic digestion of food waste: Impacts of digestate on plant growth and rhizosphere bacterial community composition and potential function in ryegrass. Biol. Fertil. Soils 2020, 56, 973–989. [Google Scholar] [CrossRef]
- Bu, J.; Wei, H.L.; Wang, Y.T.; Cheng, J.R.; Zhu, M.J. Biochar boosts dark fermentative H2 production from sugarcane bagasse by selective enrichment/colonization of functional bacteria and enhancing extracellular electron transfer. Water Res. 2021, 202, 117440. [Google Scholar] [CrossRef] [PubMed]
- Plaimart, J.; Acharya, K.; Mrozik, W.; Davenport, R.J.; Vinitnantharat, S.; Werner, D. Coconut husk biochar amendment enhances nutrient retention by suppressing nitrification in agricultural soil following anaerobic digestate application. Environ. Pollut. 2021, 268, 115684. [Google Scholar] [CrossRef]
- Xu, H.; Cai, A.; Wu, D.; Liang, G.; Xiao, J.; Xu, M.; Colinet, G.; Zhang, W. Effects of biochar application on crop productivity, soil carbon sequestration, and global warming potential controlled by biochar C:N ratio and soil pH: A global meta-analysis. Soil Tillage Res. 2021, 213, 105125. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, X.; Jiang, L.; Li, M.; Du, Z.; Zhou, G.; Shao, J.; Wang, X.; Xu, Z.; Bai, S.H.; et al. Effects of biochar application on soil greenhouse gas fluxes: A meta-analysis. GCB Bioenergy 2017, 9, 743–755. [Google Scholar] [CrossRef]
- Greenberg, I.; Kaiser, M.; Gunina, A.; Ledesma, P.; Polifka, S.; Wiedner, K.; Mueller, C.W.; Glaser, B.; Ludwig, B. Substitution of mineral fertilizers with biogas digestate plus biochar increases physically stabilized soil carbon but not crop biomass in a field trial. Sci. Total Environ. 2019, 680, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Opatokun, S.A.; Yousef, L.F.; Strezov, V. Agronomic assessment of pyrolysed food waste digestate for sandy soil management. J. Environ. Manag. 2017, 187, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, L.; He, N.; Gong, D.; Gao, H.; Ma, Z.; Fu, L.; Zhao, M.; Wang, H.; Wang, C.; et al. Soil bacterial community as impacted by addition of rice straw and biochar. Sci. Rep. 2021, 11, 22185. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Ye, R.; Yin, Y.; Sun, X.; Ma, H.; Gao, R. Reductive soil disinfestation with biochar amendment modified microbial community composition in soils under plastic greenhouse vegetable production. Soil Tillage Res. 2022, 218, 105323. [Google Scholar] [CrossRef]
- Jia, R.; Qu, Z.; You, P.; Qu, D. Effect of biochar on photosynthetic microorganism growth and iron cycling in paddy soil under different phosphate levels. Sci. Total Environ. 2017, 612, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; De la Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M.P. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Siles, J.A.; Cajthaml, T.; García-Romera, I.; Tlustoš, P.; Száková, J. Effect of digestate and fly ash applications on soil functional properties and microbial communities. Eur. J. Soil Biol. 2015, 71, 1–12. [Google Scholar] [CrossRef]
- Gielnik, A.; Pechaud, Y.; Huguenot, D.; Cébron, A.; Riom, J.M.; Guibaud, G.; Esposito, G.; van Hullebusch, E.D. Effect of digestate application on microbial respiration and bacterial communities’ diversity during bioremediation of weathered petroleum hydrocarbons contaminated soils. Sci. Total Environ. 2019, 670, 271–281. [Google Scholar] [CrossRef]
- Andruschkewitsch, M.; Wachendorf, C.; Wachendorf, M. Effects of digestates from different biogas production systems on above and belowground grass growth and the nitrogen status of the plant-soil-system. Grass Sci. 2013, 59, 183–195. [Google Scholar] [CrossRef]
- Gryndler, M.; Larsen, J.; Hršelová, H.; Řezáčová, V.; Gryndlerová, H.; Kubát, J. Organic and mineral fertilization, respectively, increase and decrease the development of external mycelium of arbuscular mycorrhizal fungi in a long-term field experiment. Mycorrhiza 2006, 16, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bamminger, C.; Poll, C.; Sixt, C.; Högy, P.; Wüst, D.; Kandeler, E.; Marhan, S. Short-term response of soil microorganisms to biochar addition in a temperate agroecosystem under soil warming. Agric. Ecosyst. Environ. 2016, 233, 308–317. [Google Scholar] [CrossRef]
- Domene, X.; Mattana, S.; Hanley, K.; Enders, A.; Lehmann, J. Medium-term effects of corn biochar addition on soil biota activities and functions in a temperate soil cropped to corn. Soil Biol. Biochem. 2014, 72, 152–162. [Google Scholar] [CrossRef]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Romano, M.; Marzaioli, R.; Baglivo, I.; Baronti, S.; Miglietta, F.; Castaldi, S. Effect of biochar addition on soil microbial community in a wheat crop. Eur. J. Soil Biol. 2014, 60, 9–15. [Google Scholar] [CrossRef]
- Imparato, V.; Hansen, V.; Santos, S.S.; Nielsen, T.K.; Giagnoni, L.; Hauggaard-Nielsen, H.; Johansen, A.; Renella, G.; Winding, A. Gasification biochar has limited effects on functional and structural diversity of soil microbial communities in a temperate agroecosystem. Soil Biol. Biochem. 2016, 99, 128–136. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zheng, J.; Zhang, B.; Lu, H.; Chi, Z.; Pan, G.; Li, L.; Zheng, J.; Zhang, X.; et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Al Seadi, T.; Lukehurst, C. Quality Management of Digestate from Biogas Plants Used as Fertiliser. International Energy Agency (IEA) Bioenergy; Task 37: Energy from Biogas. 2012. Available online: http://www.iea-biogas.net (accessed on 22 January 2024).
- Vitti, A.; Elshafie, H.S.; Logozzo, G.; Marzario, S.; Scopa, A.; Camele, I.; Nuzzaci, M. Physico-chemical characterization and biological activities of a digestate and a more stabilized digestate-derived compost from agro-waste. Plants 2021, 10, 386. [Google Scholar] [CrossRef]
- Pulvirenti, A.; Ronga, D.; Zaghi, M.; Tomasselli, A.R.; Mannella, L.; Pecchioni, N. Pelleting is a successful method to eliminate the presence of Clostridium spp. from the digestate of biogas plants. Biomass Bioenergy 2015, 81, 479–482. [Google Scholar] [CrossRef]
- Gerber, M.D.; Lucia, T.; Correa, L.; Neto, J.E.P.; Correa, É.K. Phytotoxicity of effluents from swine slaughterhouses using lettuce and cucumber seeds as bioindicators. Sci. Total Environ. 2017, 592, 86–90. [Google Scholar] [CrossRef]
- Di Maria, F.; Sordi, A.; Cirulli, G.; Gigliotti, G.; Massaccesi, L.; Cucina, M. Co-treatment of fruit and vegetable waste in sludge digesters. An analysis of the relationship among bio-methane generation, process stability and digestate phytotoxicity. Waste Manag. 2014, 34, 1603–1608. [Google Scholar] [CrossRef]
- Pastorelli, R.; Valboa, G.; Lagomarsino, A.; Fabiani, A.; Simoncini, S.; Zaghi, M.; Vignozzi, N. Recycling biogas digestate from energy crops: Effects on soil properties and crop productivity. Appl. Sci. 2021, 11, 750. [Google Scholar] [CrossRef]
- Tampio, E.; Laaksonen, I.; Rimhanen, K.; Honkala, N.; Laakso, J.; Soinne, H.; Rasa, K. Co-digestion of cattle manure with carbon-rich feedstocks: Process performance, carbon retention in soils and fertilizer value of the digestate. Sci. Total Environ. 2024; submitted. [Google Scholar]
- Baudo, R. Report on the International Interlaboratory Comparison on the Phytotoxkit. 2012. Available online: https://www.microbiotests.com/wp-content/uploads/2019/04/Report-on-the-Phytotoxkit-Interlaboratory-Comparison.pdf (accessed on 22 January 2024).
- Paradelo, R.; Villada, A.; González, D.; Barral, M.T. Evaluation of the toxicity of heavy metals and organic compounds in compost by means of two germination-elongation tests. Fresenius Environ. Bull. 2010, 19, 956–962. [Google Scholar]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; Agriculture Handbook; United States Department of Agriculture, U.S. Government Printing Office: Washington, DC, USA, 1999.
- Lagomarsino, A.; Valagussa, M.; Becagli, C.; Rocchi, F.; Tosca, A.; Pastorelli, R. Effectiveness of biochar in the short-term abatement of GHGs and NH3 emissions from digestate and slurry. Environ. Qual. Manag. 2024; submitted. [Google Scholar]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Costello, E.K.; Berg-Lyons, D.; Gonzalez, A.; Stombaugh, J.; Knights, D.; Gajer, P.; Ravel, J.; Fierer, N.; et al. Moving pictures of the human microbiome. Genome Biol. 2011, 12, R50. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.H.; Cheng, C.H.; Cheng, L.H.; Liang, C.M.; Lin, C.Y. Application of Clostridium-specific PCR primers on the analysis of dark fermentation hydrogen-producing bacterial community. Int. J. Hydrogen Energy 2008, 33, 1586–1592. [Google Scholar] [CrossRef]
- Green, T.R.; Popa, R. Turnover of carbohydrate-rich vegetal matter during microaerobic composting and after amendment in soil. Appl. Biochem. Biotechnol. 2011, 165, 270–278. [Google Scholar] [CrossRef]
- Zwielehner, J.; Liszt, K.; Handschur, M.; Lassl, C.; Lapin, A.; Haslberger, A.G. Combined PCR-DGGE fingerprinting and quantitative-PCR indicates shifts in fecal population sizes and diversity of Bacteroides; bifidobacteria and Clostridium cluster IV in institutionalized elderly. Exp. Gerontol. 2009, 44, 440–446. [Google Scholar] [CrossRef]
- Denman, S.E.; Tomkins, N.W.; McSweeney, C.S. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microb. Ecol. 2007, 62, 313–322. [Google Scholar] [CrossRef]
- Kolb, S.; Knief, C.; Stubner, S.; Conrad, R. Quantitative detection of methanotrophs in soil by novel pmoA-targeted real-time PCR assays. Appl. Environ. Microbiol. 2003, 69, 2423–2429. [Google Scholar] [CrossRef]
- Xu, M.; Schnorr, J.; Keibler, B.; Simon, H.M. Comparative analysis of 16S rRNA and amoA genes from archaea selected with organic and inorganic amendments in enrichment culture. Appl. Environ. Microbiol. 2012, 78, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- Throbäck, I.N.; Enwall, K.; Jarvis, Å.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tiquia, S.M.; Tam, N.F.Y.; Hodgkiss, I.J. Effects of composting on phytotoxicity of spent pig-manure sawdust litter. Environ. Pollut. 1996, 93, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, P.; Mourinha, C.; Farto, M.; Palma, P.; Sengo, J.; Morais, M.C.; Chuna-Queda, C. Ecotoxicological assessment of the potential impact on soil porewater, surface and groundwater from the use of organic wastes as soil amendments. Ecotoxicol. Environ. Saf. 2016, 126, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Celletti, S.; Bergamo, A.; Benedetti, V.; Pecchi, M.; Patuzzi, F.; Basso, D.; Baratieri, M.; Cesco, S.; Mimmo, T. Phytotoxicity of hydrochars obtained by hydrothermal carbonization of manure-based digestate. J. Environ. Manag. 2021, 280, 111635. [Google Scholar] [CrossRef]
- Albero, B.; Sánchez-Argüello, P.; Martín-Esteban, A.; Tampio, E.; Laaksonen, I.; Pérez, R.A. Analysis of organic contaminants and in vitro cytotocixity to test the suitability of external organic matter processing. Waste Manag. 2024; submitted. [Google Scholar]
- Fuentes, A.; Lloréns, M.; Sáez, J.; Aguilar, M.I.; Ortuño, J.F.; Meseguer, V.F. Phytotoxicity and heavy metals speciation of stabilised sewage sludges. J. Hazard. Mater. 2004, 108, 161–169. [Google Scholar] [CrossRef]
- Wüst, P.K.; Horn, M.A.; Drake, H.L. Clostridiaceae and Enterobacteriaceae as active fermenters in earthworm gut content. ISME J. 2011, 5, 92–106. [Google Scholar] [CrossRef]
- Goberna, M.; Podmirseg, S.M.; Waldhuber, S.; Knapp, B.A.; García, C.; Insam, H. Pathogenic bacteria and mineral N in soils following the land spreading of biogas digestates and fresh manure. Appl. Soil Ecol. 2011, 49, 18–25. [Google Scholar] [CrossRef]
- Bonetta, S.; Ferretti, E.; Bonetta, S.; Fezia, G.; Carraro, E. Microbiological contamination of digested products from anaerobic co-digestion of bovine manure and agricultural by-products. Lett. Appl. Microbiol. 2011, 53, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Blasco, L.; Kahala, M.; Ervasti, S.; Tampio, E. Dynamics of microbial community in response to co-feedstock composition in anaerobic digestion. Bioresour. Technol. 2022, 364, 128039. [Google Scholar] [CrossRef]
- Schnurer, A.; Jarvis, A. Microbiological Handbook for Biogas Plants; Swedish Waste Management U: Malmö, Sweden, 2010; pp. 1–74. [Google Scholar]
- Wiegel, J.; Tanner, R.A.L.P.H.; Rainey, F.A. An introduction to the family Clostridiaceae. In Prokaryotes, 3rd ed.; Dworkin, M., Falkow, S., Rosemberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 4, pp. 654–678. [Google Scholar]
- Zhu, M.; Zhang, L.; Zheng, L.; Zhuo, Y.; Xu, J.; He, Y. Typical soil redox processes in pentachlorophenol polluted soil following biochar addition. Front. Microbiol. 2018, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Obia, A.; Mulder, J.; Hale, S.E.; Nurida, N.L.; Cornelissen, G. The potential of biochar in improving drainage, aeration and maize yields in heavy clay soils. PLoS ONE 2018, 13, e0196794. [Google Scholar] [CrossRef] [PubMed]
- Prévoteau, A.; Ronsse, F.; Cid, I.; Boeckx, P.; Rabaey, K. The electron donating capacity of biochar is dramatically underestimated. Sci. Rep. 2016, 6, 32870. [Google Scholar] [CrossRef]
- Chacón, F.J.; Cayuela, M.L.; Roig, A.; Sánchez-Monedero, M.A. Understanding, measuring and tuning the electrochemical properties of biochar for environmental applications. Rev. Environ. Sci. Biotechnol. 2017, 16, 695–715. [Google Scholar] [CrossRef]
- Gomez, J.D.; Denef, K.; Stewart, C.E.; Zheng, J.; Cotrufo, M.F. Biochar addition rate influences soil microbial abundance and activity in temperate soils. Eur. J. Soil Sci. 2014, 65, 28–39. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C.; Graber, E.R. Biochar effects on the abundance, activity and diversity of the soil biota. In Biochar for Environmental Management: Science, Technology and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge, Taylor & Francis: London, UK, 2015; Volume 2, pp. 327–389. [Google Scholar]
- Xu, H.J.; Wang, X.H.; Li, H.; Yao, H.Y.; Su, J.Q.; Zhu, Y.G. Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ. Sci. Technol. 2014, 48, 9391–9399. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.N.; Gleeson, D.B.; Solaiman, Z.M.; Jones, D.L.; Murphy, D.V. Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 2012, 354, 311–324. [Google Scholar] [CrossRef]
- Devereux, R.C.; Sturrock, C.J.; Mooney, S.J. The effects of biochar on soil physical properties and winter wheat growth. Earth Environ. Sci. Trans. R. Soc. Edinb. 2012, 103, 13–18. [Google Scholar] [CrossRef]
- Barnes, R.T.; Gallagher, M.E.; Masiello, C.A.; Liu, Z.; Dugan, B. Biochar-induced changes in soil hydraulic conductivity and dissolved nutrient fluxes constrained by laboratory experiments. PLoS ONE 2014, 9, e108340. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Turunen, M.; Hyväluoma, J.; Keskinen, R.; Kaseva, J.; Nikama, J.; Reunamo, A.; Rasa, K. Pore structure of wastewater sludge chars and their water retention impacts in different soils. Biosyst. Eng. 2021, 206, 6–18. [Google Scholar] [CrossRef]
- Rasa, K.; Heikkinen, J.; Hannula, M.; Arstila, K.; Kulju, S.; Hyväluoma, J. How and why does willow biochar increase a clay soil water retention capacity? Biomass Bioenergy 2018, 119, 346–353. [Google Scholar] [CrossRef]
- Ji, M.; Zhou, L.; Zhang, S.; Luo, G.; Sang, W. Effects of biochar on methane emission from paddy soil: Focusing on DOM and microbial communities. Sci. Total Environ. 2020, 743, 140725. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, Z.-F.; Pan, X.-W.; Tan, J.-Y.; Yang, S.-S.; Wu, J.-T.; Chen, C.; Yuan, Y.; Ren, N.-Q. Sewage sludge derived biochar for environmental improvement: Advances, challenges, and solutions. Water Res. 2023, 18, 100167. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.J.; Giannopoulos, G.; Pretty, J.; Baggs, E.M.; Richardson, D.J. Biological sources and sinks of nitrous oxide and strategies to mitigate emissions. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Attard, E.; Recous, S.; Chabbi, A.; De Berranger, C.; Guillaumaud, N.; Labreuche, J.; Philippot, L.; Schmid, B.; Le Roux, X. Soil environmental conditions rather than denitrifier abundance and diversity drive potential denitrification after changes in land uses. Glob. Change Biol. 2011, 17, 1975–1989. [Google Scholar] [CrossRef]


| Cattle Manure | Digestate R1 | Digestate R2 | Biochar | ||
|---|---|---|---|---|---|
| Total solid (TS) | % | 23.9 | 12.5 | 12.9 | 98.3 |
| Volatile solid (VS) | % (TS) | 91.3 | 83.5 | 85.4 | 29.8 |
| Carbon (C) | % (TS) | 48.0 | 46.0 | 46.1 | 29.8 |
| Hydrogen (H) | % (TS) | 5.8 | 4.8 | 5.3 | 0.3 |
| Nitrogen (N) | % (TS) | 2.2 | 2.0 | 2.0 | 2.4 |
| Sulfur (S) | % (TS) | 0.3 | 0.3 | 0.3 | 1.6 |
| Oxygen (O) | % (TS) | 34.9 | 30.3 | 31.6 | −2.2 1 |
| Ash | % (TS) | 8.7 | 16.5 | 14.6 | 68.1 |
| Phosphorous (P) | g/kg TS | 3.6 | 3.1 | 2.7 | 53.7 |
| Potassium (K) | g/kg TS | 15.5 | 38.7 | 38.7 | 2.2 |
| Calcium (Ca) | mg/kg TS | 6.5 | 7.7 | 8.5 | 31.3 |
| Iron (Fe) | mg/kg TS | 0.8 | 1.5 | 2.4 | 246.0 |
| Magnesium (Mg) | mg/kg TS | 4.7 | 5.3 | 5.3 | 3.6 |
| Sodium (Na) | mg/kg TS | 2.3 | 1.9 | 1.7 | 1.1 |
| Ammonium nitrogen (NH4-N) | g/kg TS | 23.0 | 8.0 | 6.20 | 0.4 |
| Total Kjeldahl nitrogen (TNK) | g/kg TS | n.d. | 23.9 | 21.69 | n.d. |
| Treatment | Soil | Digestate R1 | Digestate R2 | Biochar | Initial Water | |
|---|---|---|---|---|---|---|
| Control soil | Control | 0.5 kg | 105 mL | |||
| Soil + 2% biochar | Bio2% | 0.5 kg | 2 g kg−1 | 105 mL | ||
| Soil + 10% Biochar | Bio10% | 0.5 kg | 10 g kg−1 | 105 mL | ||
| Soil + digestate from pilot R1 | Dig1 | 0.5 kg | 3.8 g kg−1 | 115 mL | ||
| Soil + digestate from pilot R1 + 2% biochar | Dig1+bio2% | 0.5 kg | 3.8 g kg−1 | 2 g kg−1 | 115 mL | |
| Soil + digestate from pilot R1 + 10% biochar | Dig1+bio10% | 0.5 kg | 3.8 g kg−1 | 10 g kg−1 | 115 mL | |
| Soil + digestate from pilot R2 | Dig2 | 0.5 kg | 4.1 g kg−1 | 115 mL | ||
| Soil + digestate from pilot R2 + 2% biochar | Dig2+bio2% | 0.5 kg | 4.1 g·kg−1 | 2 g kg−1 | 115 mL | |
| Soil + digestate from pilot R2 + 10% biochar | Dig2+bio10% | 0.5 kg | 4.1 g·kg−1 | 10 g kg−1 | 115 mL |
| Target Group | Target Gene | Primers | Sequences | Annealing Temperature | Reference |
|---|---|---|---|---|---|
| Bacteria | 16S rRNA | Bac341F | 5′-CCTACGGGAGGCAGCAG-3′ | 60 °C | [42] |
| Bac805R | 5′-GGACTACHVGGGTWTCTAA-3′ | [43] | |||
| Clostridiaceae | 16S rRNA | CHIS150F | 5′-AAAGGRAGATTAATACCGCATAA-3′ | 55 °C | [44] |
| cluster I and II | CLOST1R | 5′-5′TTCTTCCTAATCTCTACGCA-3′ | [45] | ||
| Clostridiaceae | 16S rRNA | CLEPF | 5′-GCACAAGCAGTGGAGT-3′ | 60 °C | [46] |
| cluster IV | CLEPR | 5′-TCCCGTAGAGTCTGG-3′ | |||
| Methanogenic | mcrA | qmcrAF | 5′-TTCGGTGGATCDCARAGRGC-3′ | 60 °C | [47] |
| archaea | qmcrAR | 5′-GBARRTCGWAWCCGTAGAAWCC-3′ | |||
| Methanotrophic | pmoA | A189F | 5′-GGNGACTGGGACTTCTGG-3′ | 60 °C | [48] |
| bacteria | Mb661R | 5′-GCCGGMGCAACGTCYTTACC-3′ | |||
| Nitrifying | amoA | AmoA1F | 5′-GGGGTTTCTACTGGTGGT-3′ | 57 °C | [49] |
| bacteria | AmoA1R | 5′-CCCCTCKGSAAAGCCTTCTTC-3′ | |||
| Nitrifying | amoA | archamoAF | 5′-STAATGGTCTGGCTTAGACG-3′ | 55 °C | [49] |
| archaea | archamoAR | 5′-GCGGCCATCCATCTGTATGT-3′ | |||
| Denitrifying | nirK | F1ACu | 5′-ATCATGGTSCTGCCGCG-3′ | 57 °C | [50] |
| bacteria | R3Cu | 5′-GCCTCGATCAGRTTGTGGTT-3′ | |||
| Denitrifying | nirS | Cd3aF1 | 5′-GTSAACGTSAAGGARACSGG-3′ | 52 °C | [50] |
| bacteria | R3Cd | 5′-GASTTCGGRTGSGTCTTGA-3′ | |||
| Denitrifying | nosZ | nosZF | 5′-CGYTGTTCHTCGACAGCCAG-3′ | 55 °C | [50] |
| bacteria | nosZ1733R | 5′-ATRTCGATCARCTGBTCGTT-3′ |
| Lactuca sativa | Triticum aestivum | |||
|---|---|---|---|---|
| Factor | F Value | p< | F Value | p< |
| Treatment | 54.9 | 0.001 | 18.1 | 0.001 |
| Dilution | 6.0 | 0.01 | 0.5 | n.s. |
| treatment × dilution | 5.8 | 0.001 | 1.2 | n.s. |
| Treatment | TC (%) | TOC (%) | TN (%) | C/N | pH | EC (dS/m) |
|---|---|---|---|---|---|---|
| Control | 1.45 (0.02) b | 0.69 (0.01) d | 0.11 (0.002) e | 13.4 (0.3) a | 7.91 (0.04) ab | 30.9 (2.8) c |
| Dig1 | 1.50 (0.05) b | 0.78 (0.01) d | 0.13 (0.002) de | 11.9 (0.2) bc | 8.00 (0.02) a | 28.5 (0.8) c |
| Dig2 | 1.59 (0.06) b | 0.79 (0.02) cd | 0.14 (0.001) cde | 11.5 (0.5) c | 7.93 (0.03) ab | 26.9 (1.6) c |
| Bio2% | 1.90 (0.01) b | 1.31 (0.01) bcd | 0.15 (0.003) bcd | 12.3 (0.3) abc | 7.87 (0.03) bc | 27.1 (1.3) d |
| Bio10% | 4.89 (0.66) a | 4.24 (0.51) a | 0.38 (0.035) a | 12.9 (0.7) ab | 7.81 (0.01) cd | 49.3 (4.9) a |
| Dig1+bio2% | 2.15 (0.12) b | 1.47 (0.17) bc | 0.17 (0.010) bc | 12.4 (0.1) abc | 7.93 (0.02) ab | 29.6 (1.7) c |
| Dig2+bio2% | 2.15 (0.13) b | 1.63 (0.24) b | 0.18 (0.016) b | 11.7 (0.4) c | 7.94 (0.03) ab | 34.1 (2.5) c |
| Dig1+bio10% | 4.31 (0.20) a | 3.68 (0.21) a | 0.34 (0.015) a | 12.7 (0.1) abc | 7.78 (0.05) d | 61.6 (3.4) b |
| Dig2+bio10% | 4.46 (0.38) a | 3.92 (0.29) a | 0.34 (0.013) a | 13.3 (0.7) a | 7.82 (0.02) cd | 61.7 (3.4) b |
| Treatment | CO2 | CH4 | N2O |
|---|---|---|---|
| Control | 3.30 (0.7) c | −0.30 (0.02) c | 0.53 (0.12) d |
| Dig1 | 4.97 (0.8) bc | −0.26 (0.04) c | 2.79 (0.27) cd |
| Dig2 | 3.97 (0.1) bc | −0.15 (0.01) b | 2.99 (0.10) cd |
| Bio2% | 7.79 (1.4) b | −0.16 (0.01) b | 1.88 (0.57) cd |
| Bio10% | 1.44 (0.1) c | −0.13 (0.01) b | 9.48 (2.1) b |
| Dig1+bio2% | 17.98 (2.8) a | −0.11 (0.01) b | 3.97 (0.19) c |
| Dig2+bio2% | 16.30 (3.2) a | −0.14 (0.01) b | 3.63 (0.69) c |
| Dig1+bio10% | 1.56 (0.1) c | −0.02 (0.01) a | 13.65 (2.33) a |
| Dig2+bio10% | 1.63 (0.3) c | −0.03 (0.01) a | 13.16 (1.94) a |
| Treatment | Total Bacteria |
|---|---|
| Control | 8.6 × 109 (6.1 × 108) bc |
| Dig1 | 1.1 × 1010 (1.1 × 109) ab |
| Dig2 | 1.3 × 1010 (2.5 × 108) a |
| Bio2% | 1.1 × 1010 (5.1 × 108) abc |
| Bio10% | 7.6 × 109 (7.2 × 108) c |
| Dig1+bio2% | 1.3 × 1010 (1.6 × 109) a |
| Dig2+bio2% | 1.1 × 1010 (1.1 × 109) ab |
| Dig1+bio10% | 1.3 × 1010 (2.4 × 109) a |
| Dig2+bio10% | 9.2 × 109 (4.7 × 108) bc |
| Nitrifiers | Methanotrophs | Methanogens | Denitrifiers | Costridiaceae | |||||
|---|---|---|---|---|---|---|---|---|---|
| Thesis | Bacterial | Archaeal | Cluster I and II | Cluster IV | |||||
| amoA | amoA | pmoA | mcrA | nirK | nirS | nosZ | 16S rDNA | 16S rDNA | |
| Control | 1.0 (0.15) ab | 1.1 (0.44) | 1.0 (0.03) a | 1.0 (0.10) b | 1.0 (0.14) b | 1.0 (0.08) | 1.0 (0.11) ab | 0.9 (0.05) bc | 1.0 (0.16) d |
| Dig1 | 0.8 (0.09) b | 2.0 (0.28) | 0.9 (0.15) ab | 54.1 (6.55) a | 1.4 (0.07) ab | 1.1 (0.04) | 1.1 (0.03) ab | 4.1 (1.06) a | 21.3 (1.15) ab |
| Dig2 | 0.8 (0.25) b | 2.0 (0.70) | 0.8 (0.06) abc | 42.0 (13.8) a | 1.2 (0.14) ab | 1.2 (0.08) | 1.0 (0.03) ab | 2.5 (1.68) ab | 15.2 (1.57) bc |
| Bio2% | 0.8 (0.15) b | 2.5 (0.47) | 0.6 (0.02) c | 1.8 (0.09) b | 1.2 (0.31) ab | 1.1 (0.11) | 1.0 (0.10) ab | 0.2 (0.18) c | 3.0 (1.25) d |
| Bio10% | 1.2 (0.22) ab | 1.2 (0.64) | 1.0 (0.08) a | 1.8 (0.30) b | 0.8 (0.02) b | 0.9 (0.03) | 1.1 (0.05) ab | 0.1 (0.03) c | 2.4 (0.36) d |
| Dig1+bio2% | 0.7 (0.08) b | 2.3 (0.29) | 0.8 (0.13) bc | 62.6 (7.07) a | 1.7 (0.74) ab | 1.1 (0.23) | 0.9 (0.08) b | 0.6 (0.50) bc | 18.1 (1.0) abc |
| Dig2+bio2% | 1.5 (0.32) a | 1.5 (0.32) | 0.7 (0.04) bc | 64.2 (5.87) a | 2.2 (0.49) a | 1.2 (0.18) | 1.1 (0.06) ab | 0.1 (0.01) c | 22.4 (0.83) a |
| Dig1+bio10% | 0.8 (0.12) b | 2.6 (1.40) | 0.7 (0.07) bc | 50.2 (12.4) a | 1.1 (0.15) b | 0.9 (0.06) | 1.0 (0.09) ab | 0.0 (0.00) c | 14.8 (3.79) c |
| Dig2+bio10% | 1.0 (0.19) b | 1.3 (0.15) | 0.8 (0.06) abc | 51.8 (11.6) a | 1.4 (0.23) ab | 1.1 (0.13) | 1.2 (0.22) a | 0.3 (0.13) c | 14.4 (4.16) bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastorelli, R.; Casagli, A.; Rocchi, F.; Tampio, E.; Laaksonen, I.; Becagli, C.; Lagomarsino, A. Effects of Anaerobic Digestates and Biochar Amendments on Soil Health, Greenhouse Gas Emissions, and Microbial Communities: A Mesocosm Study. Appl. Sci. 2024, 14, 1917. https://doi.org/10.3390/app14051917
Pastorelli R, Casagli A, Rocchi F, Tampio E, Laaksonen I, Becagli C, Lagomarsino A. Effects of Anaerobic Digestates and Biochar Amendments on Soil Health, Greenhouse Gas Emissions, and Microbial Communities: A Mesocosm Study. Applied Sciences. 2024; 14(5):1917. https://doi.org/10.3390/app14051917
Chicago/Turabian StylePastorelli, Roberta, Alessandro Casagli, Filippo Rocchi, Elina Tampio, Ilmari Laaksonen, Claudia Becagli, and Alessandra Lagomarsino. 2024. "Effects of Anaerobic Digestates and Biochar Amendments on Soil Health, Greenhouse Gas Emissions, and Microbial Communities: A Mesocosm Study" Applied Sciences 14, no. 5: 1917. https://doi.org/10.3390/app14051917