The Effect of Red Potato Pulp Preparation and Stage of Its Incorporation into Sourdough or Dough on the Quality and Health-Promoting Value of Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Red Potato Preparations
2.2. Preparation of the Sourdough
2.3. Determination of pH and Total Titratable Acidity of the Dough
2.4. Wheat–Rye Bread Production
2.5. Bread Quality Analysis
2.5.1. Loaf Mass and Bread Yield and Baking Loss
2.5.2. Total Titratable Acidity of Bread Crumb
2.5.3. Bread Volume
2.5.4. Moisture of the Bread Crumb
2.5.5. Analysis of Texture Parameters
2.5.6. Color Analysis
2.6. Analysis of Basic Chemical Composition of Bread and Preparations
2.7. Antioxidants Content and Antiradical Activity of Bread and Preparations
2.7.1. Extraction Procedure
2.7.2. Total Polyphenol Content
2.7.3. Content of Phenolic Acids, Flavonols, and Anthocyanins
2.7.4. Content of Flavonoids
2.7.5. Antioxidant Activity
2.8. Molecular Parameters of Polysaccharide Matrix in Bread
2.8.1. Sample Preparation
2.8.2. Chromatographic Analysis
2.8.3. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Obtained Preparations
3.2. Characterization of Sourdough, Dough, and Bread with the Participation of the Tested Preparations in Terms of Changes in Acidity
3.3. Quality of Bread Made Using Preparations
3.4. Chemical Composition of Investigated Breads
3.5. Polyphenol Content and Antioxidant Activity of Bread Supplemented with Preparations Extruded from Red Potato Pulp
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Meral, R.; Köse, Y.E. The effect of bread-making process on the antioxidant activity and phenolic profile of enriched breads. Qual. Assur. Saf. Crops Foods 2019, 11, 171–181. [Google Scholar] [CrossRef]
- Škrbić, B.; Filipčev, B. Nutritional and sensory evaluation of wheat breads supplemented with oleic-rich sunflower seed. Food Chem. 2008, 108, 119–129. [Google Scholar] [CrossRef]
- Swieca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Czyż, J. The influence of protein-flavonoid interactions on protein digestibility in vitro and the antioxidant quality of breads enriched with onion skin. Food Chem. 2013, 141, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Sluková, M.; Jurkaninová, L.; Švec, I.; Skřivan, P. Rye—The nutritional and technological evaluation in Czech cereal technology—A review: Grain and flours. Czech J. Food Sci. 2021, 39, 3–8. [Google Scholar] [CrossRef]
- Fernández-Peláez, J.; Paesani, C.; Gómez, M. Sourdough Technology as a Tool for the Development of Healthier Grain-Based Products: An Update. Agronomy 2020, 10, 1962. [Google Scholar] [CrossRef]
- Taglieri, I.; Macaluso, M.; Bianchi, A.; Sanmartin, C.; Quartacci, M.F.; Zinnai, A.; Venturi, F. Overcoming bread quality decay concerns: Main issues for bread shelf life as a function of biological leavening agents and different extra ingredients used in formulation. A review. J. Sci. Food Agric. 2021, 101, 1732–1743. [Google Scholar] [CrossRef]
- Torrieri, E.; Pepe, O.; Ventorino, V.; Masi, P.; Cavella, S. Effect of sourdough at different concentrations on quality and shelf life of bread. LWT Food Sci. Technol. 2014, 56, 508–516. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L. Lactobacilli in sourdough fermentation. Food Res. Int. 2007, 40, 539–558. [Google Scholar] [CrossRef]
- Altunkaya, A.; Hedegaard, R.V.; Brimer, L.; Gökmen, V.; Skibsted, L.H. Antioxidant capacity versus chemical safety of wheat bread enriched with pomegranate peel powder. Food Funct. 2013, 4, 722–727. [Google Scholar] [CrossRef]
- Filipčev, B.; Lević, L.; Bodroža-Solarov, M.; Mišljenović, N.; Koprivica, G. Quality Characteristics and Antioxidant Properties of Breads Supplemented with Sugar Beet Molasses-Based Ingredients. Int. J. Food Prop. 2010, 13, 1035–1053. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.-H.; Abdul Aziz, N.A.; Azahari, B. Physico-chemical characteristics and sensory evaluation of wheat bread partially substituted with banana (Musa acuminata × balbisiana cv. Awak) pseudo-stem flour. Food Chem. 2013, 139, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-L.; Hurang, S.-L.; Chen, W.; Weng, Y.-M.; Tseng, C.-Y. Qualities and antioxidant properties of bread as affected by the incorporation of yam flour in the formulation. Int. J. Food Sci. Technol. 2004, 39, 231–238. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Cheng, K.-W.; Jiang, Y.; Chen, F.; Wang, M. The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem. 2010, 119, 49–53. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Perera, C.O.; Waterhouse, G.I.N. Application of FT-IR and Raman spectroscopy for the study of biopolymers in breads fortified with fibre and polyphenols. Food Res. Int. 2013, 50, 574–585. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Perera, C.O.; Waterhouse, G.I.N. Exploring the interactions between blackcurrant polyphenols, pectin and wheat biopolymers in model breads; a FTIR and HPLC investigation. Food Chem. 2012, 131, 802–810. [Google Scholar] [CrossRef]
- Zhu, F.; Sun, J. Physicochemical and sensory properties of steamed bread fortified with purple sweet potato flour. Food Biosci. 2019, 30, 100411. [Google Scholar] [CrossRef]
- Gumul, D.; Korus, J.; Ziobro, R.; Kruczek, M. Enrichment of wheat bread with apple pomace as a way to increase pro-health constituents. Qual. Assur. Saf. Crops Foods 2019, 11, 231–240. [Google Scholar] [CrossRef]
- Gumul, D.; Korus, J.; Surma, M.; Ziobro, R. Pulp obtained after isolation of starch from red and purple potatoes (Solanum tuberosum L.) as an innovative ingredient in the production of gluten-free bread. PLoS ONE 2020, 15, e0229841. [Google Scholar] [CrossRef]
- Sharma, H.K.; Kumar, N. Utilization of Carrot Pomace. In Food Processing By-Products and Their Utilization; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 207–229. ISBN 978-1-118-43292-1. [Google Scholar]
- Santos, D.; Pintado, M.; Lopes da Silva, J.A. Potential nutritional and functional improvement of extruded breakfast cereals based on incorporation of fruit and vegetable by-products—A review. Trends Food Sci. Technol. 2022, 125, 136–153. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Food industry by-products used as functional ingredients of bakery products. Trends Food Sci. Technol. 2017, 67, 106–128. [Google Scholar] [CrossRef]
- Brown, C.R.; Wrolstad, R.; Durst, R.; Yang, C.P.; Clevidence, B. Breeding Studies in Potatoes Containing High Concentrations of Anthocyanins. Am. J. Potato Res. Off. Publ. Potato Assoc. Am. 2003, 80, 241–249. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Šulc, M.; Orsák, M.; Pivec, V.; Hejtmánková, A.; Dvořák, P.; Čepl, J. Cultivar differences of total anthocyanins and anthocyanidins in red and purple-fleshed potatoes and their relation to antioxidant activity. Food Chem. 2009, 114, 836–843. [Google Scholar] [CrossRef]
- Nara, K.; Miyoshi, T.; Honma, T.; Koga, H. Antioxidative activity of bound-form phenolics in potato peel. Biosci. Biotechnol. Biochem. 2006, 70, 1489–1491. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.F.; Miller, J.C.; Cisneros-Zevallos, L. Antioxidant capacity, anthocyanins and total phenolics in purple-and red-fleshed potato (Solanum tuberosum L.) genotypes. Am. J. Potato Res. 2005, 82, 271–277. [Google Scholar] [CrossRef]
- Makarova, E.; Górnaś, P.; Konrade, I.; Tirzite, D.; Cirule, H.; Gulbe, A.; Pugajeva, I.; Seglina, D.; Dambrova, M. Acute anti-hyperglycaemic effects of an unripe apple preparation containing phlorizin in healthy volunteers: A preliminary study. J. Sci. Food Agric. 2015, 95, 560–568. [Google Scholar] [CrossRef]
- Rodríguez-Muela, C.; Rodríguez, H.E.; Arzola, C.; Díaz-Plascencia, D.; Ramírez-Godínez, J.A.; Flores-Mariñelarena, A.; Mancillas-Flores, P.F.; Corral, G. Antioxidant activity in plasma and rumen papillae development in lambs fed fermented apple pomace. J. Anim. Sci. 2015, 93, 2357–2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef] [PubMed]
- PN-A-74108:1996; Bread—Research Methods. Polski Komitet Normalizacyjny: Warsaw, Poland, 1996. Available online: https://sklep.pkn.pl/pn-a-74108-1996p.html (accessed on 12 January 2023).
- Official Methods of Analysis of AOAC International—18th Edition, Revision 3. Available online: https://www.techstreet.com/standards/official-methods-of-analysis-of-aoac-international-18th-edition-revision-3?product_id=1678986 (accessed on 15 February 2021).
- International Commission on Illumination. Colorimetry, 3rd ed.; Commission Internationale de l’Eclairage: Vienna, Austria, 2004. [Google Scholar]
- Gumul, D.; Kruczek, M.; Ivanišová, E.; Słupski, J.; Kowalski, S. Apple Pomace as an Ingredient Enriching Wheat Pasta with Health-Promoting Compounds. Foods 2023, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, Phenolics, and Color of Cabernet Franc, Merlot, and Pinot Noir Wines from British Columbia. J. Agric. Food Chem. 1999, 47, 4009–4017. [Google Scholar] [CrossRef]
- Oomah, B.D.; Cardador-Martínez, A.; Loarca-Piña, G. Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L). J. Sci. Food Agric. 2005, 85, 935–942. [Google Scholar] [CrossRef]
- El Hariri, B.; Sallé, G.; Andary, C. Involvement of flavonoids in the resistance of two poplar cultivars to mistletoe (Viscum album L.). Protoplasma 1991, 162, 20–26. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Neukom, H.; Rutz, W. Observations on starch retrogradation and bread stuffing. Lebensm. Wiss. Technol. Food Sci. Technol. 1981, 14, 292–295. [Google Scholar]
- Lukasiewicz, M.; Kowalski, S. Low power microwave-assisted enzymatic esterification of starch. Starch Stärke 2012, 64, 188–197. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Wolska, L.; Bartoszek, A.; Namiesnik, J. Charakterystyka polifenoli: Wystepowanie, wlasciwosci, przeglad metod analitycznych. Bromatol. Chem. Toksykol. 2005, 38, 81–92. [Google Scholar]
- Gallardo, C.; Jiménez, L.; García-Conesa, M.-T. Hydroxycinnamic acid composition and in vitro antioxidant activity of selected grain fractions. Food Chem. 2006, 3, 455–463. [Google Scholar] [CrossRef]
- Kita, A.; Bąkowska-Barczak, A.; Hamouz, K.; Kułakowska, K.; Lisińska, G. The effect of frying on anthocyanin stability and antioxidant activity of crisps from red- and purple-fleshed potatoes (Solanum tuberosum L.). J. Food Compos. Anal. 2013, 2, 169–175. [Google Scholar] [CrossRef]
- Nemś, A.; Pęksa, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kita, A.; Drożdż, W.; Hamouz, K. Anthocyanin and antioxidant activity of snacks with coloured potato. Food Chem. 2015, 172, 175–182. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients 2020, 12, 1118. [Google Scholar] [CrossRef] [Green Version]
- Casado, A.; Álvarez, A.; González, L.; Fernández, D.; Marcos, J.L.; Tornadijo, M.E. Effect of fermentation on microbiological, physicochemical and physical characteristics of sourdough and impact of its use on bread quality. Czech J. Food Sci. 2017, 35, 496–506. [Google Scholar] [CrossRef] [Green Version]
- Gänzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef]
- Lopez, H.W.; Krespine, V.; Guy, C.; Messager, A.; Demigne, C.; Remesy, C. Prolonged Fermentation of Whole Wheat Sourdough Reduces Phytate Level and Increases Soluble Magnesium. J. Agric. Food Chem. 2001, 49, 2657–2662. [Google Scholar] [CrossRef]
- Banu, I.; Vasilean, I.; Constantin, O.E.; Aprodu, I. Prediction of rye dough behaviour and bread quality using response surface methodology. Ir. J. Agric. Food Res. 2011, 50, 239–247. [Google Scholar]
- Stear, C.A. Handbook of Breadmaking Technology; Elsevier: London, UK, 1990. [Google Scholar]
- Gänzle, M.; Ripari, V. Composition and function of sourdough microbiota: From ecological theory to bread quality. Int. J. Food Microbiol. 2016, 239, 19–25. [Google Scholar] [CrossRef]
- König, H.; Unden, G.; Fröhlich, J. Biology of Microorganisms on Grapes, in Must and in Wine, 2nd ed.; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-86760-1. [Google Scholar]
- Bjerrum, N. Zur Theorie der chemischen Reaktionsgeschwindigkeit. Z. Phys. Chem. 1924, 108, 82–100. [Google Scholar] [CrossRef]
- Ortiz de Erive, M.; Wang, T.; He, F.; Chen, G. Development of high-fiber wheat bread using microfluidized corn bran. Food Chem. 2020, 310, 125921. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Guo, Q.; Wang, C.; Ding, H.H.; Cui, S.W. Fenugreek fibre in bread: Effects on dough development and bread quality. LWT Food Sci. Technol. 2016, 71, 274–280. [Google Scholar] [CrossRef]
- Özkaya, B.; Baumgartner, B.; Özkaya, H. Effects of concentrated and dephytinized wheat bran and rice bran addition on bread properties. J. Texture Stud. 2018, 49, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Najafi, M.A.; Haddadi, T. Effect of fermentation process, wheat bran size and replacement level on some characteristics of wheat bran, dough, and high-fiber Tafton bread. J. Cereal Sci. 2019, 85, 56–61. [Google Scholar] [CrossRef]
- Witczak, M.; Korus, J.; Ziobro, R.; Juszczak, L. The effects of maltodextrins on gluten-free dough and quality of bread. J. Food Eng. 2010, 96, 258–265. [Google Scholar] [CrossRef]
- Miyazaki, M.; Maeda, T.; Morita, N. Effect of various dextrin substitutions for wheat flour on dough properties and bread qualities. Food Res. Int. 2004, 1, 59–65. [Google Scholar] [CrossRef]
- Bialowas, B.; Szymanowski, K. Cutting forces during drilling and selected physical and mechanical properties of the finish coating based on epoxy resin. Ann. Wars. Univ. Life Sci. SGGW For. Wood Technol. 2020, 111, 106–115. [Google Scholar] [CrossRef]
- Hutchings, J.B. Food Colour and Appearance; Springer: New York, NY, USA, 1994; Volume 513. [Google Scholar]
- Pasqualone, A.; Laddomada, B.; Centomani, I.; Paradiso, V.M.; Minervini, D.; Caponio, F.; Summo, C. Bread making aptitude of mixtures of re-milled semolina and selected durum wheat milling by-products. LWT Food Sci. Technol. 2017, 78, 151–159. [Google Scholar] [CrossRef]
- Taranto, F.; Delvecchio, L.N.; Mangini, G.; Faro, L.D.; Blanco, A.; Pasqualone, A. Molecular and physico-chemical evaluation of enzymatic browning of whole meal and dough in a collection of tetraploid wheats. J. Cereal Sci. 2012, 3, 405–414. [Google Scholar] [CrossRef]
- Aguirre, J.F.; Osella, C.A.; Carrara, C.R.; Sánchez, H.D.; Buera, M.d.P. Effect of storage temperature on starch retrogradation of bread staling. Starch Stärke 2011, 63, 587–593. [Google Scholar] [CrossRef]
- Ding, S.; Yang, J. The effects of sugar alcohols on rheological properties, functionalities, and texture in baked products—A review. Trends Food Sci. Technol. 2021, 111, 670–679. [Google Scholar] [CrossRef]
- Arendt, E.K.; Ryan, L.A.M.; Dal Bello, F. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef]
- Guha, M.; Zakiuddin Ali, S. Molecular Degradation of Starch During Extrusion Cooking of Rice. Int. J. Food Prop. 2002, 5, 509–521. [Google Scholar] [CrossRef]
- Mihhalevski, A.; Heinmaa, I.; Traksmaa, R.; Pehk, T.; Mere, A.; Paalme, T. Structural Changes of Starch during Baking and Staling of Rye Bread. J. Agric. Food Chem. 2012, 60, 8492–8500. [Google Scholar] [CrossRef]
- De Vuyst, L.; Comasio, A.; Kerrebroeck, S.V. Sourdough production: Fermentation strategies, microbial ecology, and use of non-flour ingredients. Crit. Rev. Food Sci. Nutr. 2021, 63, 2447–2479. [Google Scholar] [CrossRef]
- Han, H.-M.; Koh, B.-K. Antioxidant activity of hard wheat flour, dough and bread prepared using various processes with the addition of different phenolic acids. J. Sci. Food Agric. 2011, 91, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value and chemical composition of pseudocereals as gluten-free ingredients. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 4), 240–257. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Stojceska, V.; Ainsworth, P.; Plunkett, A.; İbanoğlu, Ş. The effect of extrusion cooking using different water feed rates on the quality of ready-to-eat snacks made from food by-products. Food Chem. 2009, 114, 226–232. [Google Scholar] [CrossRef]
- Maillard, M.-N.; Berset, C. Evolution of Antioxidant Activity during Kilning: Role of Insoluble Bound Phenolic Acids of Barley and Malt. J. Agric. Food Chem. 1995, 43, 1789–1793. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Wang, L.; Huber, G.M.; Pitts, N.L. Effect of baking on dietary fibre and phenolics of muffins incorporated with apple skin powder. Food Chem. 2008, 107, 1217–1224. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.; Perera, C.O. Properties of Bread Dough with Added Fiber Polysaccharides and Phenolic Antioxidants: A Review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef] [Green Version]
- Renard, C.M.; Baron, A.; Guyot, S.; Drilleau, J.F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Rohn, S.; Rawel, H.M.; Kroll, J. Inhibitory effects of plant phenols on the activity of selected enzymes. J. Agric. Food Chem. 2002, 50, 3566–3571. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Li, S.; Gao, W. Preparation, physicochemical characterization and in vitro digestibility on solid complex of maize starches with quercetin. LWT Food Sci. Technol. 2011, 44, 787–792. [Google Scholar] [CrossRef]



| Bread Type | Sourdough | Wheat Flour | Rye Flour | Water | Baker’s Yeast | Salt | Preparation |
|---|---|---|---|---|---|---|---|
| (g) | |||||||
| SB | 289 (rye flour) | 200 | 40 | 131 | 2 | 8 | - |
| E0PP_T1 | 289 (rye flour) | 190 | 30 | 131 | 2 | 8 | 20 (E0PP) |
| E30PP_T1 | 289 (rye flour) | 190 | 30 | 131 | 2 | 8 | 20 (E30PP) |
| E40PP_T1 | 289 (rye flour) | 190 | 30 | 131 | 2 | 8 | 20 (E40PP) |
| E0PP_T2 | 289 (rye flour), 30 (E0PP) | 190 | 50 | 131 | 2 | 8 | - |
| E30PP_T2 | 289 (rye flour), 30 (E30PP) | 190 | 50 | 131 | 2 | 8 | - |
| E40PP_T2 | 289 (rye flour), 30 (E40PP) | 190 | 50 | 131 | 2 | 8 | - |
| Sample name | Chemical Composition | |||||||
| Protein | Fat | Starch | Ash | Carbohydrates | ||||
| (g/100 g DM) | ||||||||
| E0PP | 0.28 c ± 0.03 | 0.39 c ± 0.01 | 89.27 a ± 0.08 | 0.12 a ± 0.00 | 10.44 a ± 0.02 | |||
| E30PP | 2.81 b ± 0.00 | 0.45 b ± 0.00 | 75.74 b ± 0.15 | 1.95 b ± 0.00 | 8.55 b ± 0.02 | |||
| E40PP | 3.64 a ± 0.04 | 0.52 a ± 0.01 | 73.66 c ± 0.08 | 2.35 c ± 0.00 | 5.67 c ± 0.02 | |||
| Dietary fiber | ||||||||
| Soluble fraction | Insoluble fraction | Total | ||||||
| (g/100 g DM) | ||||||||
| E0PP | 0.02 c ± 0.02 | 0 c ± 0 | 0.02 c ± 0.02 | |||||
| E30PP | 1.09 b ± 0.05 | 0.70 b ± 0.04 | 1.79 b ± 0.01 | |||||
| E40PP | 1.21 a ± 0.02 | 1.14 a ± 0.03 | 2.35 a ± 0.05 | |||||
| Total phenolic content—with F-C (mg catechin/100 g DM) | Total phenolic content—without F-C (mg catechin/100 g DM) | ABTS (mMTx/kg DM) | ||||||
| E0PP | 73.95 c ± 0.54 | 8.58 c ± 0.43 | 13.67 c ± 0.02 | |||||
| E30PP | 905.98 b ± 1.6 | 651.26 b ± 2.02 | 70.48 b ± 0.67 | |||||
| E40PP | 1135.69 a ± 1.63 | 807.93 a ± 1.26 | 78.33 a ± 0.41 | |||||
| The other phenolic compounds | ||||||||
| Phenolic acids (mg gallic acid/100 g DM) | Flavonols (mg quercetin/100 g DM) | Anthocyanins (mg cyanidin-3-glucoside/100 g DM) | Flavonoids (mg rutin/100 g DM) | |||||
| E0PP | 0.00 c ± 0.00 | 0.00 c ± 0.00 | 0.00 c ± 0.00 | 1.22 c ± 0.07 | ||||
| E30PP | 89.50 b ± 2.82 | 31.31 b ± 1.74 | 2.64 b ± 0.06 | 266.44 b ± 0.91 | ||||
| E40PP | 116.6 a ± 6.40 | 45.93 a ± 2.00 | 4.10 a ± 0.01 | 358.56 a ± 1.03 | ||||
| Type of Sourdough | pH | TTA [mL 1 M NaOH/100 g Sample] | ||||
|---|---|---|---|---|---|---|
| 0 | 24 h | 48 h | 0 | 24 h | 48 h | |
| standard | 6.11 cC ± 0.02 | 3.96 aB ± 0.05 | 3.89 aA ± 0.04 | 1.02 aA ± 0.12 | 10.31 aB ± 0.38 | 14.64 aC ± 0.65 |
| E0PP | 6.10 cC ± 0.02 | 3.98 aB ± 0.01 | 3.83 aA ± 0.01 | 1.73 abA ± 0.10 | 9.84 aB ± 0.14 | 14.77 abC ± 0.29 |
| E30PP | 5.85 bC ± 0.01 | 3.95 aB ± 0.01 | 3.89 aA ± 0.01 | 1.71 bA ± 0.03 | 9.87 aB ± 0.04 | 15.18 abC ± 0.58 |
| E40PP | 5.78 aC ± 0.01 | 4.00 aB ± 0.01 | 3.85 aA ± 0.01 | 2.27 cA ± 0.10 | 11.89 bB ± 0.03 | 15.93 bC ± 0.21 |
| Type of Dough and Bread | Initial pH of Dough | pH of Dough after Fermentation | Initial TTA of Dough | Dough TTA after Fermentation | TTA of Bread Crumb |
|---|---|---|---|---|---|
| mL 1 M NaOH/100 g Sample | |||||
| SB | 4.33 b ± 0.01 | 4.28 c ± 0.01 | 6.65 bc ± 0.65 | 7.11 c ± 0.18 | 5.95 b ± 0.26 |
| E0PP_T1 | 4.29 c ± 0.00 | 4.28 c ± 0.01 | 7.39 a ± 0.47 | 8.13 b ± 0.07 | 5.96 b ± 0.01 |
| E30PP_T1 | 4.32 b ± 0.01 | 4.35 a ± 0.01 | 6.65 bc ± 0.37 | 8.62 ab ± 0.13 | 6.49 a ± 0.15 |
| E40PP_T1 | 4.37 a ± 0.01 | 4.33 ab ± 0.01 | 6.27 c ± 0.38 | 8.04 b ± 0.42 | 6.60 a ± 0.0 |
| E0PP_T2 | 4.38 a ± 0.01 | 4.34 a ± 0.01 | 5.86 c ± 0.23 | 6.84 c ± 0.46 | 6.59 a ± 0.0 |
| E30PP_T2 | 4.32 b ± 0.01 | 4.34 a ± 0.01 | 6.77 bc ± 0.15 | 8.98 a ± 0.07 | 6.50 a ± 0.14 |
| E40PP_T2 | 4.37 a ± 0.01 | 4.31 b ± 0.01 | 6.32 c ± 0.06 | 8.62 ab ± 0.41 | 6.10 b ± 0.15 |
| Type of Bread | Weight of Loaf (g) | Volume (cm3) | Yield (%) | Baking Loss (%) |
|---|---|---|---|---|
| SB | 83.7 a ± 0.9 | 198.13 b ± 1.83 | 140.26 a ± 1.51 | 16.26 b ± 0.90 |
| E0PP_T1 | 82.98 ab ± 0.52 | 192.16 d ± 1.91 | 139.00 ab ± 0.88 | 17.01 ab ± 0.52 |
| E30PP_T1 | 83.04 ab ± 0.67 | 189.21 e ± 2.25 | 139.10 ab ± 1.13 | 16.96 ab ± 0.68 |
| E40PP_T1 | 83.11 ab ± 0.32 | 189.39 e ± 0.89 | 139.22 ab ± 0.53 | 16.89 ab ± 0.32 |
| E0PP_T2 | 82.59 b ± 0.24 | 206.68 a ± 1.16 | 138.33 b ± 0.40 | 17.41 a ± 0.24 |
| E30PP_T2 | 83.25 ab ± 0.53 | 197.89 b ± 0.77 | 139.45 ab ± 0.89 | 16.75 ab ± 0.53 |
| E40PP_T2 | 83.05 ab ± 0.11 | 195.17 c ± 2.16 | 139.10 ab ± 0.19 | 16.95 ab ± 0.11 |
| Type of Bread | Crumb | Crust | ||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | L* | a* | b* | ΔE | |
| SB | 60.70 a ± 0.39 | 4.00 f ± 0.03 | 19.25 de ± 0.06 | - | 52.62 b ± 0.32 | 16.82 b ± 0.19 | 36.82 a ± 0.14 | - |
| E0PP_T1 | 59.27 b ± 0.35 | 4.14 e ± 0.05 | 19.42 d ± 0.14 | 1.46 c ± 0.33 | 48.18 c ± 0.27 | 17.54 a ± 0.09 | 33.60 d ± 0.15 | 5.53 d ± 0.31 |
| E30PP_T1 | 53.43 c ± 0.16 | 7.23 c ± 0.04 | 24.62 b ± 0.06 | 9.60 b ± 0.11 | 45.06 e ± 0.14 | 14.37 c ± 0.16 | 25.19 e ± 0.40 | 14.09 a ± 0.41 |
| E40PP_T1 | 50.99 d ± 0.30 | 7.96 b ± 0.06 | 25.46 a ± 0.14 | 12.19 d ± 0.17 | 43.91 f ± 1.10 | 17.54 a ± 0.52 | 30.09 c ± 0.43 | 11.04 b ± 1.16 |
| E0PP_T2 | 60.71 a ± 0.24 | 3.97 f ± 0.06 | 19.13 e ± 0.15 | 0.28 d ± 0.09 | 55.96 a ± 0.29 | 9.78 d ± 0.08 | 24.71 f ± 0.17 | 14.40 a ± 0.19 |
| E30PP_T2 | 53.37 c ± 0.34 | 7.10 d ± 0.05 | 24.09 c ± 0.30 | 9.32 b ± 0.37 | 43.81 f ± 0.50 | 17.16 b ± 0.39 | 29.03 d ± 0.48 | 11.78 b ± 0.56 |
| E40PP_T2 | 50.82 d ± 0.32 | 8.04 a ± 0.05 | 24.74 b ± 0.12 | 12.01 d ± 0.21 | 47.02 d ± 1.04 | 15.88 c ± 0.39 | 28.94 d ± 0.56 | 9.75 c ± 0.85 |
| Type of Preparation Used | Crumb Moisture (g/100 g) | ||
|---|---|---|---|
| Baking Day | After 24 h | After 48 h | |
| SB | 43.14 Ab ± 0.51 | 41.75 aA ± 0.36 | 41.20 aA ± 0.14 |
| E0PP_T1 | 42.34 bB ± 0.32 | 40.50 cA ± 0.44 | 40.20 bA ± 0.08 |
| E30PP_T1 | 42.44 bC ± 0.23 | 40.96 bcB ± 0.26 | 39.07 cA ± 0.02 |
| E40PP_T1 | 42.37 bB ± 0.40 | 41.72 aB ± 0.40 | 40.11 bA ± 1.09 |
| E0PP_T2 | 41.54 cC ± 0.35 | 40.96 bcB ± 0.11 | 40.03 bA ± 0.34 |
| E30PP_T2 | 41.53 cB ± 0.29 | 41.37 abB ± 0.10 | 40.60 abA ± 0.21 |
| E40PP_T2 | 42.05 bcB ± 0.38 | 41.85 aA ± 0.24 | 41.15 aA ± 0.21 |
| Type of Bread | Hardness (N) | Cohesiveness (-) | ||||
|---|---|---|---|---|---|---|
| Baking Day | After 24 h | After 48 h | Baking Day | After 24 h | After 48 h | |
| SB | 10.20 abA ± 1.27 | 19.98 cdB ± 0.09 | 27.16 aC ± 0.12 | 0.77 abC ± 0.02 | 0.60 abB ± 0.01 | 0.52 abA ± 0.0 |
| E0PP_T1 | 11.34 aA ± 1.12 | 22.45 aAB ± 0.30 | 30.79 aB ± 8.25 | 0.75 cC ± 0.01 | 0.61 abB ± 0.01 | 0.51 abA ± 0.01 |
| E30PP_T1 | 10.50 aA ± 0.90 | 20.65 bB ± 0.14 | 24.80 aC ± 0.02 | 0.79 bcC ± 0.01 | 0.63 bB ± 0.00 | 0.51 abA ± 0.02 |
| E40PP_T1 | 9.81 abcA ± 0.14 | 21.09 abB ± 0.26 | 26.97 aB ± 3.78 | 0.80 aC ± 0.01 | 0.62 aB ± 0.02 | 0.53 aA ± 0.02 |
| E0PP_T2 | 8.10 cA ± 0.12 | 15.42 dB ± 0.59 | 23.24 aC ± 1.07 | 0.77 abcC ± 0.00 | 0.58 bB ± 0.02 | 0.49 abA ± 0.03 |
| E30PP_T2 | 8.08 cA ± 0.46 | 18.52 cB ± 0.80 | 22.51 aB ± 2.05 | 0.79 abC ± 0.00 | 0.59 abB ± 0.00 | 0.51 abA ± 0.00 |
| E40PP_T2 | 8.52 bcA ± 0.01 | 19.73 bcB ± 1.30 | 23.18 aC ± 0.19 | 0.78 abcC ± 0.00 | 0.60 abB ± 0.01 | 0.47 bA ± 0.03 |
| Type of Bread | Protein | Fat | Ash | Dietary Fiber | ||
|---|---|---|---|---|---|---|
| Insoluble | Soluble | Total | ||||
| (g/100 g DM) | ||||||
| SB | 10.69 d ± 0.03 | 1.92 a ± 0.02 | 2.77 c ± 0.01 | 2.49 e ± 0.06 | 2.28 f ± 0.03 | 4.77 g ± 0.03 |
| E0PP_T1 | 10.68 d ± 0.11 | 1.96 a ± 0.03 | 2.76 c ± 0.02 | 2.68 d ± 0.03 | 2.41 c ± 0.02 | 5.09 f ± 0.02 |
| E30PP_T1 | 10.73 cd ± 0.05 | 1.72 bc ± 0.02 | 2.81 b ± 0.00 | 2.98 c ± 0.02 | 2.87 b ± 0.01 | 5.84 d ± 0.02 |
| E40PP_T1 | 10.86 b ± 0.02 | 1.96 a ± 0.04 | 2.87 a ± 0.00 | 3.46 b ± 0.02 | 3.07 a ± 0.01 | 6.53 b ± 0.01 |
| E0PP_T2 | 10.93 da ± 0.01 | 1.68 c ± 0.05 | 2.78 c ± 0.00 | 2.75 d ± 0.02 | 2.48 c ± 0.05 | 5.22 e ± 0.03 |
| E30PP_T2 | 10.84 bc ± 0.04 | 1.89 a ± 0.02 | 2.82 b ± 0.01 | 3.08 c ± 0.05 | 2.87 b ± 0.05 | 5.95 c ± 0.00 |
| E40PP_T2 | 10.83 bc ± 0.02 | 1.78 b ± 0.02 | 2.92 a ± 0.00 | 3.55 a ± 0.02 | 3.14 b ± 0.04 | 6.69 a ± 0.02 |
| Sample | Mn·10−3 | Mw·10−5 | ƉƉ |
|---|---|---|---|
| (g·mol−1) | |||
| E0PP | 61.78 | 11.39 | 18.4 |
| E30PP | 51.13 | 8.73 | 17.1 |
| E40PP | 52.69 | 8.12 | 15.4 |
| SB | 47.28 | 9.20 | 19.45 |
| E0PP_T1 | 45.71 | 9.70 | 21.20 |
| E30PP_T1 | 35.00 | 8.71 | 24.85 |
| E40PP_T1 | 36.41 | 8.64 | 23.75 |
| E0PP_T2 | 58.42 | 10.57 | 18.15 |
| E30PP_T2 | 35.69 | 8.65 | 24.30 |
| E40PP_T2 | 34.81 | 8.70 | 25.0 |
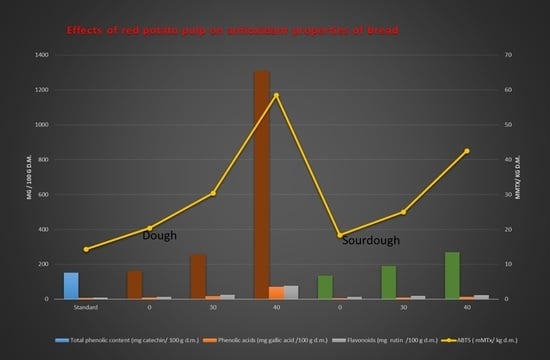
| Type of Bread | Total Phenolic Content (mg Catechin/100 g DM) | ABTS (mMTx/kg DM) | |
|---|---|---|---|
| with F-C | without F-C | ||
| SB | 151.82 f ± 0.77 | 149.49 de ± 2.44 | 14.31 f ± 0.00 |
| E0 PP_T1 | 161.51 e ± 2.33 | 147.76 e ± 9.77 | 20.35 e ± 1.20 |
| E30PP_T1 | 255.18 c ± 0.0 | 223.75 b ± 9.77 | 30.39 c ± 1.64 |
| E40PP_T1 | 1308.36 a ± 0.0 | 975.07 a ± 7.33 | 58.48 a ± 1.06 |
| E0PP_T2 | 133.85 g ± 0.78 | 137.39 e ± 0.0 | 18.33 e ± 1.08 |
| E30PP_T2 | 189.72 d ± 1.55 | 163.30 d ± 2.45 | 24.97 d ± 0.76 |
| E40PP_T2 | 269.41 b ± 3.10 | 204.76 c ± 2.44 | 42.52 b ± 1.28 |
| Type of Bread | Phenolic Acids (mg Gallic Acid/100 g DM) | Flavonols (mg Quercetin/100 g DM) | Anthocyanins (mg Cyanidin-3-Glucoside/100 g DM) | Flavonoids (mg Rutin/100 g DM) |
|---|---|---|---|---|
| SB | 7.16 e ± 0.33 | 2.11 c ± 0.40 | 1.8 b ± 1.1 | 8.04 e ± 0.0 |
| E0PP_T1 | 8.35 de ± 0.68 | 0.00 d ± 0.0 | 0.0 c ± 0.0 | 12.69 d ± 0.60 |
| E30PP_T1 | 15.76 b ± 1.01 | 6.94 b ± 0.81 | 8.0 a ± 1.1 | 24.94 b ± 1.20 |
| E40PP_T1 | 70.24 a ± 0.34 | 24.87 a ± 1.20 | 0.0 c ± 0.0 | 76.91 a± 2.98 |
| E0PP_T2 | 5.24 f ± 0.34 | 0.97 cd ± 0.40 | 0.0 c ± 0.0 | 12.69 d ± 0.60 |
| E30PP_T2 | 8.59 d ± 0.34 | 1.82 c ± 0.0 | 0.0 c ± 0.0 | 19.03 c ± 1.20 |
| E40PP_T2 | 13.13 c ± 0.0 | 5.80 b ± 0.81 | 2.6 b ± 0.0 | 22.41 b ± 1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litwinek, D.; Gumul, D.; Łukasiewicz, M.; Zięba, T.; Kowalski, S. The Effect of Red Potato Pulp Preparation and Stage of Its Incorporation into Sourdough or Dough on the Quality and Health-Promoting Value of Bread. Appl. Sci. 2023, 13, 7670. https://doi.org/10.3390/app13137670
Litwinek D, Gumul D, Łukasiewicz M, Zięba T, Kowalski S. The Effect of Red Potato Pulp Preparation and Stage of Its Incorporation into Sourdough or Dough on the Quality and Health-Promoting Value of Bread. Applied Sciences. 2023; 13(13):7670. https://doi.org/10.3390/app13137670
Chicago/Turabian StyleLitwinek, Dorota, Dorota Gumul, Marcin Łukasiewicz, Tomasz Zięba, and Stanisław Kowalski. 2023. "The Effect of Red Potato Pulp Preparation and Stage of Its Incorporation into Sourdough or Dough on the Quality and Health-Promoting Value of Bread" Applied Sciences 13, no. 13: 7670. https://doi.org/10.3390/app13137670