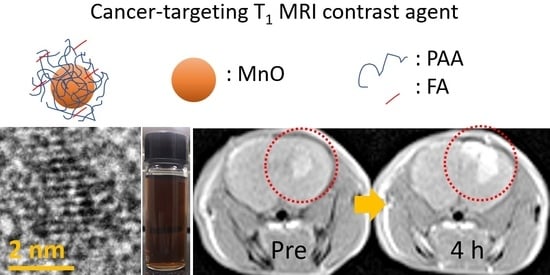
In Vivo Positive Magnetic Resonance Imaging of Brain Cancer (U87MG) Using Folic Acid-Conjugated Polyacrylic Acid-Coated Ultrasmall Manganese Oxide Nanoparticles
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of FA-PAA
2.3. Preparation of FA-PAA-Coated MnO Nanoparticles
2.4. Characterizations
2.5. Relaxometric Property Measurements
2.6. Cellular Toxicity Measurements
2.7. Preparation of Cancer Model Nude Mice
2.8. In Vivo T1 MRI Experiments
3. Results
3.1. Particle Size, Colloidal Stability, and Crystal Structure
3.2. Surface-Coating
3.3. Magnetic Properties
3.4. In Vitro Cell Viabilities
3.5. Water Proton Spin Relaxivity Values
3.6. In Vivo T1 MRI
4. Discussion
5. Conclusions
- (1)
- The PAA coating conferred good colloidal stability and low cellular cytotoxicity, while FA conferred cancer-targeting ability on the nanoparticles.
- (2)
- The FA-PAA-coated MnO nanoparticles exhibited a high value of r1 = 9.3 s−1mM−1 (r2/r1 = 2.2) owing to the strong interactions between the numerous Mn2+ species on the nanoparticle surfaces and the many water molecules around the nanoparticles attracted by PAA.
- (3)
- The effectiveness of the FA-PAA-coated MnO nanoparticles as a cancer-targeting T1 MRI contrast agent was proved by the strong positive contrast enhancements of the brain cancer site compared to those of the normal brain tissue.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Haris, M.; Yadav, S.K.; Rizwan, A.; Singh, A.; Wang, E.; Hariharan, H.; Reddy, R.; Marincola, F.M. Molecular magnetic resonance imaging in cancer. J. Transl. Med. 2015, 13, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harisinghani, M.G.; O’Shea, A.; Weissleder, R. Advances in clinical MRI technology. Sci. Transl. Med. 2019, 18, eaba2591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.-D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S.-K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Caravan, P. Strategies for increasing the sensitivity of gadolinium-based MRI contrast agents. Chem. Soc. Rev. 2006, 35, 512–523. [Google Scholar] [CrossRef]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef]
- Lauffer, R.B. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: Theory and design. Chem. Rev. 1987, 87, 901–927. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; David, A.E.; Chertok, B.; Zhang, L.; Yu, F.; Yang, V.C. Magnetic nanoparticles for MRI of brain tumors. Curr. Pharm. Biotechnol. 2012, 13, 2403–2416. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Baek, M.J.; Choi, E.S.; Woo, S.; Kim, J.H.; Kim, T.J.; Jung, J.C.; Chae, K.S.; Chang, Y.; Lee, G.H. Paramagnetic ultrasmall gadolinium oxide nanoparticles as advanced T1 MRI contrast agent: Account for large longitudinal relaxivity, optimal particle diameter, and in vivo T1 MR images. ACS Nano 2009, 3, 3663–3669. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Schmieder, A.H.; Wickline, S.A.; Lanza, G.M. Manganese-based MRI contrast agents: Past, present, and future. Tetrahedron 2011, 67, 8431–8444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, Z.; Xie, J. Development of manganese-based nanoparticles as contrast probes for magnetic resonance imaging. Theranostics 2012, 2, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Sako, S.; Umemura, Y.; Ohshima, K.; Sakai, M.; Bandow, S. Magnetic property of antiferromagnetic MnO ultrafine-particle. J. Phys. Soc. Jpn. 1996, 65, 280–284. [Google Scholar] [CrossRef]
- Trohidou, K.N.; Zianni, X.; Blackman, J.A. Surface effects on the magnetic behaviour of antiferromagnetic particles. J. Appl. Phys. 1998, 84, 2795–2800. [Google Scholar] [CrossRef]
- Silva, A.C.; Lee, J.H.; Aoki, I.; Koretsky, A.P. Manganese-enhanced magnetic resonance imaging (MEMRI): Methodological and practical considerations. NMR Biomed. 2004, 17, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.A.; Allagadda, V.M.; Leamon, C.P. Targeting therapeutic and imaging agents to folate receptor positive tumors. Curr. Pharm. Biotechnol. 2005, 6, 131–150. [Google Scholar] [CrossRef]
- Zhao, X.; Li, H.; Lee, R.J. Targeted drug delivery via folate receptors. Expert Opin. Drug Deliv. 2008, 5, 309–319. [Google Scholar] [CrossRef]
- Leamon, C.P.; Reddy, J.A. Folate-targeted chemotherapy. Adv. Drug Deliv. Rev. 2004, 56, 1127–1141. [Google Scholar] [CrossRef]
- Xia, W.; Low, P.S. Folate-targeted therapies for cancer. J. Med. Chem. 2010, 53, 6811–6824. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Shao, C.; Qu, Y.; Li, S.; Gu, W.; Zheng, T.; Ye, L.; Yu, C. Folic acid-conjugated MnO nanoparticles as a T1 contrast agent for magnetic resonance imaging of tiny brain gliomas. ACS Appl. Mater. Interfaces 2014, 6, 19850–19857. [Google Scholar] [CrossRef]
- Rana, S.; Shetake, N.G.; Barick, K.C.; Pandey, B.N.; Salunke, H.G.; Hassan, P.A. Folic acid conjugated Fe3O4 magnetic nanoparticles for targeted delivery of doxorubicin. Dalton Trans. 2016, 45, 17401–17408. [Google Scholar] [CrossRef]
- Li, L.; Gao, F.; Jiang, W.; Wu, X.; Cai, Y.; Tangs, J.; Gao, X.; Gao, F. Folic acid-conjugated superparamagnetic iron oxide nanoparticles for tumor-targeting MR imaging. Drug Deliv. 2016, 23, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.P.; Mathur, R.; Singh, G.; Kaul, A.; Bag, N.; Singh, S.; Kumar, H.; Patra, M.; Mishra, A.K. Evaluation of folate conjugated superparamagnetic iron oxide nanoparticles for scintigraphic/magnetic resonance imaging. J. Biomed. Nanotechnol. 2013, 9, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Quarta, A.; Curcio, A.; Kakwere, H.; Pellegrino, T. Polymer coated inorganic nanoparticles: Tailoring the nanocrystal surface for designing nanoprobes with biological implications. Nanoscale 2012, 4, 3319–3334. [Google Scholar] [CrossRef]
- Larson, N.; Ghandehari, H. Polymeric conjugates for drug delivery. Chem. Mater. 2012, 24, 840–853. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-L.; Lee, C.-F.; Chiu, W.-Y. Preparation and properties of poly(acrylic acid) oligomer stabilized superparamagnetic ferrofluid. J. Colloid Interface Sci. 2005, 291, 411–420. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Martin, D.A.; Alvarez, V.A.; Gonzalez, J.S. Polyacrylic acid-coated iron oxide magnetic nanoparticles: The polymer molecular weight influence. Colloid Surf. A 2018, 543, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Liufu, S.; Xiao, H.; Li, Y. Adsorption of poly(acrylic acid) onto the surface of titanium dioxide and the colloidal stability of aqueous suspension. J. Colloid Interface Sci. 2005, 281, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Henoumont, C.; Laurent, S.; Elst, L.V. How to perform accurate and reliable measurements of longitudinal and transverse relaxation times of MRI contrast media in aqueous solutions. Contrast Media Mol. Imaging 2009, 4, 312–321. [Google Scholar] [CrossRef]
- Moore, T.E.; Ellis, M.; Selwood, P.W. Solid oxides and hydroxides of manganese. J. Am. Chem. Soc. 1950, 72, 856–866. [Google Scholar] [CrossRef]
- Mendive, C.B.; Bredow, T.; Blesa, M.A.; Bahnemann, D.W. ATR-FTIR measurements and quantum chemical calculations concerning the adsorption and photoreaction of oxalic acid on TiO2. Phys. Chem. Chem. Phys. 2006, 8, 3232–3247. [Google Scholar] [CrossRef]
- Daecon, G.B.; Philips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Benoit, D.N.; Zhu, H.; Lilierose, M.H.; Verm, R.A.; Ali, N.; Morrison, A.N.; Fortner, J.D.; Avendano, C.; Colvin, V.L. Measuring the grafting density of nanoparticles in solution by analytical ultracentrifugation and total organic carbon analysis. Anal. Chem. 2012, 84, 9238–9245. [Google Scholar] [CrossRef] [Green Version]
- Lide, D.R. CRC Handbook of Chemistry and Physics; Internet Version; CRC Press: Boca Raton, FL, USA, 2005; pp. 4–68. [Google Scholar]
- Baek, M.J.; Park, J.Y.; Xu, W.; Kattel, K.; Kim, H.G.; Lee, E.J.; Patel, A.K.; Lee, J.J.; Chang, Y.; Kim, T.J.; et al. Water-soluble MnO nanocolloid for a molecular T1 MR imaging: A facile one-pot synthesis, in vivo T1 MR images, and account for relaxivities. ACS Appl. Mater. Interfaces 2010, 2, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Ho, S.L.; Tegafaw, T.; Cha, H.; Chang, Y.; Oh, I.T.; Yaseen, A.M.; Marasini, S.; Ghazanfari, A.; Yue, H.; et al. Stable and non-toxic ultrasmall gadolinium oxide nanoparticle colloids (coating material = polyacrylic acid) as high-performance T1 magnetic resonance imaging contrast agents. RSC Adv. 2018, 8, 3189–3197. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.-J.; Liu, S.; Yue, H.; Park, J.A.; Cha, H.; Ho, S.L.; Marasini, S.; Ghazanfari, A.; Ahmad, M.Y.; Miao, X.; et al. Hydrophilic biocompatible poly(acrylic acid-co-maleic acid) polymer as a surface-coating ligand of ultrasmall Gd2O3 nanoparticles to obtain a high r1 value and T1 MR images. Diagnostics 2021, 11, 2. [Google Scholar] [CrossRef]
- Ahmad, M.Y.; Ahmad, M.W.; Yue, H.; Ho, S.L.; Park, J.A.; Jung, K.-H.; Cha, H.; Marasini, S.; Ghazanfari, A.; Liu, S.; et al. In vivo positive magnetic resonance imaging applications of poly(methyl vinyl ether-alt-maleic acid)-coated ultra-small paramagnetic gadolinium oxide nanoparticles. Molecules 2020, 25, 1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.; Anisur, R.M.; Ko, M.K.; Im, G.H.; Lee, J.H.; Lee, I.S. Hollow manganese oxide nanoparticles as multifunctional agents for magnetic resonance imaging and drug delivery. Angew. Chem. Int. Ed. 2009, 48, 321–324. [Google Scholar] [CrossRef]
- Kim, T.; Momin, E.; Choi, J.; Yuan, K.; Zaidi, H.; Kim, J.; Park, M.; Lee, N.; McMahon, M.T.; Quinones-Hinojosa, A.; et al. Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T1 contrast agents for labeling and MRI tracking of adipose derived mesenchymal stem cells. J. Am. Chem. Soc. 2011, 133, 2955–2961. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, J.; Chen, K.; Bu, L.; Lee, S.; Cheng, Z.; Li, X.; Chen, X. HSA coated MnO nanoparticles with prominent MRI contrast for tumor imaging. Chem. Commun. 2010, 46, 6684–6686. [Google Scholar] [CrossRef] [Green Version]
- Marfavi, Z.H.; Farhadi, M.; Jameie, S.B.; Zahmatkeshan, M.; Pirhajati, V.; Jameie, M. Glioblastoma U-87MG tumour cells suppressed by ZnO folic acid-conjugated nanoparticles: An in vitro study. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2783–2790. [Google Scholar] [CrossRef] [PubMed]










| Particle Dimension (nm) | Surface-Coating Amount | Magnetic Properties | Water Proton Spin Relaxivity (s−1mM−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| davg 1 | aavg 2 | ξavg 3 (mV) | P 4 (wt%) | σ 5 (1/nm) | N 6 | Magnetism | Net magnetization (emu/g) | r1 | r2 | |
| 100 K | 300 K | |||||||||
| 2.7 ± 0.1 | 22.2 ± 0.1 | −32.6 ± 0.4 | 44.6 | 0.6 | 14 | Paramagnetic | 3.2 | 1.1 | 9.3 ± 0.1 | 20.4 ± 0.4 |
| Core | Coating Ligand or Chelate | davg (nm) | r1 (s−1mM−1) | Applied Field (T) | Ref. |
|---|---|---|---|---|---|
| MnO nanoparticle | PEG 1-phospholipid | 20 | 0.21 | 3.0 | [39] |
| MnO nanoparticle | Mesoporous SiO2 | 15 | 0.65 | 11.7 | [40] |
| MnO nanoparticle | Human serum albumin | 25.2 | 1.97 | 7.0 | [41] |
| MnO nanoparticle | FA-TETT 2 | 16.8 | 4.83 | 7.0 | [20] |
| MnO nanoparticle | D-glucuronic acid | 2.5 | 7.02 | 1.5 | [35] |
| MnO nanoparticle | FA-PAA 3 | 2.7 | 9.3 | 3.0 | This study |
| Mn2+ ion | EDTA 4 | - | 2.9 | 0.47 | [8] |
| Gd3+ ion | DTPA 5 | - | 4.1 | 0.47 | [8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marasini, S.; Yue, H.; Ho, S.-L.; Park, J.-A.; Kim, S.; Yang, J.-U.; Cha, H.; Liu, S.; Tegafaw, T.; Ahmad, M.Y.; et al. In Vivo Positive Magnetic Resonance Imaging of Brain Cancer (U87MG) Using Folic Acid-Conjugated Polyacrylic Acid-Coated Ultrasmall Manganese Oxide Nanoparticles. Appl. Sci. 2021, 11, 2596. https://doi.org/10.3390/app11062596
Marasini S, Yue H, Ho S-L, Park J-A, Kim S, Yang J-U, Cha H, Liu S, Tegafaw T, Ahmad MY, et al. In Vivo Positive Magnetic Resonance Imaging of Brain Cancer (U87MG) Using Folic Acid-Conjugated Polyacrylic Acid-Coated Ultrasmall Manganese Oxide Nanoparticles. Applied Sciences. 2021; 11(6):2596. https://doi.org/10.3390/app11062596
Chicago/Turabian StyleMarasini, Shanti, Huan Yue, Son-Long Ho, Ji-Ae Park, Soyeon Kim, Ji-Ung Yang, Hyunsil Cha, Shuwen Liu, Tirusew Tegafaw, Mohammad Yaseen Ahmad, and et al. 2021. "In Vivo Positive Magnetic Resonance Imaging of Brain Cancer (U87MG) Using Folic Acid-Conjugated Polyacrylic Acid-Coated Ultrasmall Manganese Oxide Nanoparticles" Applied Sciences 11, no. 6: 2596. https://doi.org/10.3390/app11062596