Biomimetic Amorphous Titania Nanoparticles as Ultrasound Responding Agents to Improve Cavitation and ROS Production for Sonodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
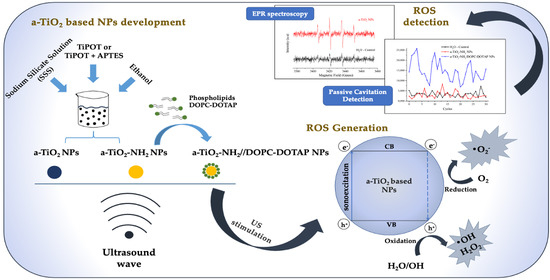
2.2. Synthesis and Functionalization of a-TiO2 NPs
2.3. Coupling of a-TiO2-NH2 NPs with DOPC-DOTAP Lipid
2.4. Physico-Chemical Characterization of a-TiO2 Based NPs
2.4.1. Transmission Electron Microscopy (TEM)
2.4.2. X-ray Powder Diffraction (XRD)
2.4.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.4.4. Ultraviolet-Visible (UV-Vis) Spectroscopy
2.4.5. Dynamic Light Scattering (DLS) and Zeta Potential (Z-Potential)
2.5. Fluorescence Microscopy Imaging
2.6. Spin Trapping Measurements Coupled with EPR Spectroscopy
2.7. Passive Cavitation Detection Experiment
3. Results
3.1. A-TiO2 NPs Structural Characterization: TEM, XRD, and FTIR
3.2. Ultraviolet-Visible (UV-Vis) Spectroscopy
3.3. Dynamic Light Scattering (DLS) and Zeta Potential (Z-Potential)
3.4. Colocalization Experiments
3.5. EPR Spectroscopy
3.6. Passive Cavitation Detection
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Cancer. Available online: www.who.int/cancer/en/ (accessed on 4 May 2020).
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef] [Green Version]
- Pitot, H. Fundamentals of Oncology; Marcel Dekker: New York, NY, USA, 1978. [Google Scholar]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46 Pt 1, 6387–6392. [Google Scholar]
- Gerlowski, L.E.; Jain, R.K. Microvascular permeability of normal and neoplastic tissues. Microvasc. Res. 1986, 31, 288–305. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Overchuk, M.; Zheng, G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials 2018, 156, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticle for Drug Delivery: Current insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barui, S.; Cauda, V. Multimodal decorations of mesoporous silica nanoparticles for improved cancer therapy. Pharmaceutics 2020, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.C. Sol-Gel Silica Nanoparticles in Medicine: A Natural Choice. Design, Synthesis and Products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef] [Green Version]
- Racca, L.; Cauda, V. Remotely-activated nanoparticles for anticancer therapy. Nano Micro Lett. 2021, 13, 1–34. [Google Scholar] [CrossRef]
- Vighetto, V.; Ancona, A.; Racca, L.; Limongi, T.; Troia, A.; Canavese, G.; Cauda, V. The synergistic effect of nanocrystals combined with ultrasound in the generation of reactive oxygen species for biomedical applications. Front. Bioeng. Biotechnol. 2019, 7, 374. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, V.; Hay, N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, L.; Chuang, C.C.; Wu, S.; Zuo, L. Reactive oxygen species in redox cancer therapy. Cancer Lett. 2015, 367, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ancona, A.; Dumontel, B.; Garino, N.; Demarco, B.; Chatzitheodoridou, D.; Fazzini, W.; Engelke, H.; Cauda, V. Lipid-Coated Zinc Oxide Nanoparticles as Innovative ROS-Generators for Photodynamic Therapy in Cancer Cells. Nanomaterials 2018, 8, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat 2019, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Masamune, K. Flexible laser endoscope for minimally invasive photodynamic diagnosis (PDD) and therapy (PDT) toward efficient tumor removal. Opt. Express 2017, 25, 16795–16812. [Google Scholar] [CrossRef]
- Rosenthal, I.; Sostaric, J.Z.; Riesz, P. Sonodynamic therapy—A review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem. 2004, 11, 349–363. [Google Scholar] [CrossRef]
- Yumita, N.; Nishigaki, R.; Umemura, K.; Umemura, S.I. Hematoporphyrin as a sensitizer of cell-damaging effect of ultrasound. Jpn. J. Cancer Res. 1989, 80, 219–222. [Google Scholar] [CrossRef]
- Son, S.; Kim, J.H.; Wang, X.; Zhang, C.; Yoon, S.A.; Shin, J.; Sharma, A.; Lee, M.H.; Cheng, L.; Wu, J.; et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020, 49, 3244–3261. [Google Scholar] [CrossRef]
- Canavese, G.; Ancona, A.; Racca, L.; Canta, M.; Dumontel, B.; Barbaresco, F.; Limongi, T.; Cauda, V. Nanoparticle-assisted ultrasound: A special focus on sonodynamic therapy against cancer. Chem. Eng. J. 2018, 340, 155–172. [Google Scholar] [CrossRef]
- Harada, Y.; Ogawa, K.; Irie, Y.; Endo, H.; Feril, L.B.; Uemura, T.; Tachibana, K. Ultrasound activation of TiO2 in melanoma tumors. J. Control. Release 2011, 149, 190–195. [Google Scholar] [CrossRef]
- Yasuda, J.; Miyashita, T.; Taguchi, K.; Yoshizawa, S.; Umemura, S.I. Quantitative assessment of reactive oxygen sonochemically generated by cavitation bubbles. Jpn. J. Appl. Phys. 2015, 54, 07KF24. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Zheng, Y.; Chen, Y. Micro/nanoparticle-augmented sonodynamic therapy (SDT): Breaking the depth shallow of photoactivation. Adv. Mater. 2016, 28, 8097–8129. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, X.; Gong, F.; Chao, Y.; Cheng, L. Newly developed strategies for improving sonodynamic therapy. Mater. Horiz. 2020, 7, 2028–2046. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Giuntini, F.; Francovich, A.; Serpe, L. The bright side of sound: Perspectives on the biomedical application of sonoluminescence. Photochem. Photobiol. Sci. 2020, 19, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, Y.; Xia, T. Black Titanium Dioxide Nanomaterials in Photocatalysis. Int. J. Photoenergy 2017, 2017, 8529851. [Google Scholar] [CrossRef] [Green Version]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Etacheri, V.; Valentin, C.D.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatlysts: Advances in theory and experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Pelaez, M.; Nolan, N.; Pillai, S.; Seery, M.; Falaras, P. A review on the visible light active titanium dioxide photocatalysis for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Hernández, S.; Cauda, V.; Chiodoni, A.; Dallorto, S.; Sacco, A.; Hidalgo, D.; Celasco, E.; Pirri, C.F. Optimization of 1D ZnO@TiO2 Core–Shell Nanostructures for Enhanced Photoelectrochemical Water Splitting under Solar Light Illumination. ACS Appl. Mater. Interfaces 2014, 6, 12153–12167. [Google Scholar] [CrossRef]
- Tianyi, W.; Haitao, J.; Long, W.; Qinfu, Z.; Tongying, J.; Bing, W.; Siling, W. Potential application of functional porous TiO2 nanoparticles in light-controlled drug release and targeted drug delivery. Acta Biomater. 2015, 13, 354–363. [Google Scholar]
- Jin, X.; Xiaobo, P.; Mengyan, W.; Jiong, M.; Yiyan, F.; Pei-Nan, W.; Lan, M. The role of surface modification for TiO2 nanoparticles in cancer cells. Colloids Surf. B Biointerfaces 2016, 143, 148–155. [Google Scholar]
- Rehman, F.U.; Zhao, C.; Jiang, H.; Wang, X. Biomedical applications of nano-titania in theranostics and photodynamic therapy. Biomater. Sci. 2016, 4, 40–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdan, J.; Plawinska-Czarnak, J.; Zarzynska, J. Nanoparticles of Titanium and Zinc Oxides as Novel Agents in Tumor Treatment: A review. Nanoscale Res. Lett. 2017, 12, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, S.; Kobayashi, H.; Narita, T.; Kanehira, K.; Sonezaki, S.; Kudo, N.; Kubota, Y.; Terasaka, S.; Houkin, K. Sonodynamic therapy using water-dispersed TiO2 polyethylene glycol compound on glioma cells: Comparison of cytotoxic mechanism with photodynamic therapy. Ultrason. Sonochem. 2011, 18, 1197–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Ranade, M.R.; Navrotsky, A.; Zhang, H.Z.; Banfield, J.F.; Elder, S.H.; Zaban, A.; Borse, P.H.; Kulkarmi, S.K.; Doran, G.S.; Whitfield, H.J. Energetics of nanocrystalline TiO2. Proc. Natl. Acad. Sci. USA 2002, 99, 6476–6481. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.Z.; Chen, B.; Banfield, J.F. Atomic structure of nanometer-sized amorphous TiO2. Phys. Rev. B 2008, 78, 214106. [Google Scholar] [CrossRef] [Green Version]
- Hoang, V.V. Atomic mechanism of vitrification process in simple monatomic nanoparticles. Eur. Phys. J. D 2011, 61, 627–635. [Google Scholar] [CrossRef]
- Hoang, V.V.; Ganguli, D. Amorphous nanoparticles—Experiments and computer simulations. Phys. Rep. 2012, 518, 81–140. [Google Scholar] [CrossRef]
- Hoang, V.V. Structural properties of simulated liquid and amorphous TiO2. Phys. Status Solid B 2007, 244, 1280–1287. [Google Scholar] [CrossRef]
- Hoang, V.V.; Zung, H.; Trong, N.H.B. Structural properties of amorphous TiO2 nanoparticles. Eur. Phys. J. D 2007, 44, 515–524. [Google Scholar] [CrossRef]
- Feldman, C.; Moorjani, K. Amorphous Semiconductors. Johns Hopkins APL Tech. Dig. 1968, 7, 2–9. [Google Scholar]
- Xiong, H.; Slater, M.D.; Balasubramaniam, M.; Johnson, C.S.; Rajh, T. Amorphous TiO2 Nanotube Anode for rechargeable sodium ion batteries. J. Phys. Chem. Lett. 2011, 2, 2560–2565. [Google Scholar] [CrossRef]
- Fritzche, H. Optical and Electrical energy gaps in amorphous semiconductors. J. Non-Cryst. Solids 1971, 6, 49–71. [Google Scholar] [CrossRef]
- Kanna, M.; Wongnawa, S.; Buddee, S.; Dilokkhunakul, K.; Pinpithak, P. Amorphous titanium dioxide: A recyclable dye remover for water treatment. J. Sol-Gel Sci. Technol. 2010, 53, 162–170. [Google Scholar] [CrossRef]
- Jeong, Y.-M.; Lee, J.-K.; Jun, H.-W.; Kim, G.-R.; Choe, Y. Preparation of super-hydrophilic amorphous titanium dioxide thin film via PECVD process and its application to dehumidifying heat exchangers. J. Ind. Eng. Chem. 2009, 15, 202–206. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, L.; Li, X.; Cao, Q.; Wang, H.; Tang, X.; Ye, L. Highly water dispersible TiO2 nanoparticles for doxorubicin delivery: Effect of loading mode on therapeutic efficacy. J. Mater. Chem. 2011, 21, 18003–18010. [Google Scholar] [CrossRef]
- Koch, S.; Kessler, M.; Mandel, K.; Dembski, K.; Heuze, S.; Hackenberg, S. Polycarboxylate ethers: The key towards non-toxic TiO2 nanoparticles stabilisation in physiological solutions. Colloid Surf. B 2016, 143, 7–14. [Google Scholar] [CrossRef]
- Matos, J.C.; Oliveira, C.; Gonçalves, M.C. Daylight Bactericidal Titania Textiles: A contribution to Nosocomial Infections Control. Molecules 2019, 24, 1891. [Google Scholar] [CrossRef] [Green Version]
- Peixoto, I.; Faria, M.; Gonçalves, M.C. Synthesis and characterization of novel integral asymmetric monophasic cellulose-acetate/silica/titania and cellulose-acetate/titania membranes. Membranes 2020, 10, 195. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Pereira, J.C.; Matos, J.C.; Vasconcelos, H.C. Photonic band gap and bactericide performance of amorphous sol-gel titania: An Alternative to crystalline TiO2. Molecules 2018, 23, 1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancona, A.; Troia, A.; Garino, N.; Dumontel, B.; Cauda, V.; Canavese, G. Leveraging re-chargeable nanobubbles on amine-functionalized ZnO nanocrystals for sustained ultrasound cavitation towards echographic imaging. Ultrason. Sonochem. 2020, 67, 105132. [Google Scholar] [CrossRef] [PubMed]
- Sviridov, A.P.; Osminkina, L.A.; Nikolaev, A.L.; Kudryavtsev, A.A.; Vasiliev, A.N.; Timoshenko, V.Y. Lowering of the cavitation threshold in aqueous suspensions of porous silicon nanoparticles for sonodynamic therapy applications. Appl. Phys. Lett. 2015, 107, 123107. [Google Scholar] [CrossRef]
- Limongi, T.; Canta, M.; Racca, L.; Ancona, A.; Vighetto, V.; Tritta, S.; Cauda, V. Improving dispersal of therapeutic nanoparticles in the human body. Nanomedicine 2019, 14, 797–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauda, V.; Engelke, H.; Sauer, A.; Arcizet, D.; Bräuchle, C.; Rädler, J.; Bein, T. Colchicine-loaded lipid bilayer-coated 50 nm mesoporous nanoparticles efficiently induce microtubule depolymerization upon cell uptake. Nano Lett. 2010, 10, 2484–2492. [Google Scholar] [CrossRef] [PubMed]
- Dumontel, B.; Canta, M.; Engelke, H.; Chiodoni, A.; Racca, L.; Ancona, A.; Limongi, T.; Canavese, G.; Cauda, V. Enhanced Biostability and Cellular Uptake of Zinc Oxide Nanocrystals Shielded with Phospholipid Bilayer. J. Mater. Chem. B 2017, 5, 8799–8813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumontel, B.; Susa, F.; Limongi, T.; Canta, M.; Racca, L.; Chiodoni, A.; Garino, N.; Chiabotto, G.; Centomo, M.L.; Pignochino, Y.; et al. ZnO nanocrystals shuttled by extracellular vesicles as effective trojan nano-horses against cancer cells. Nanomedicine 2019, 14, 2815–2833. [Google Scholar] [CrossRef] [Green Version]
- Illes, B.; Hirschle, P.; Barnert, S.; Cauda, V.; Wuttke, S.; Engelke, H. Exosome-Coated Metal Organic Framework Nanoparticles: An Efficient Drug Delivery Platform. Chem. Mater. 2017, 29, 8042–8046. [Google Scholar] [CrossRef]
- Ploetz, E.; Zimpel, A.; Cauda, V.; Bauer, D.; Lamb, D.C.; Haisch, C.; Zahler, S.; Vollmar, A.M.; Wuttke, S.; Engelke, H. Metal-Organic Framework Nanoparticles Induce Pyroptosis in Cells Controlled by the Extracellular pH. Adv. Mater. 2020, 32, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, M.C. Nanomaterials. In Materials for Construction and Civil Engineering: Science, Processing, and Design; Gonçalves, M.C., Margarido, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 629–677. [Google Scholar]
- Lei, Y.; Zhang, L.D.; Fan, J.C. Fabrication, characterization and Raman study of TiO2 nanowire arrays prepared by anodic oxidative hydrolysis of TiCl3. Chem. Phys. Lett. 2001, 338, 231–236. [Google Scholar] [CrossRef]
- Mogyorosi, K.; Dekany, I.; Fendler, J.H. Preparation and characterization of clay mineral intercalated titanium dioxide nanoparticles. Langmuir 2003, 19, 2938–2946. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Xu, P.; Zhao, X.; Liu, Y.; Chao, S. The effect of properties of semiconductor oxide films on photocatalytic decomposition of dyeing wastewater. Thin Solid Films 1998, 334, 103–108. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, S.; Fu, X.C. The growth of TiO2-x film and the quantum size effect studied by UV-Vis spectroscopy, SEM, TEM and AB initio calculation. J. Phys. Chem. Solids 1998, 59, 1647. [Google Scholar]
- Hema, M.; Arasi, A.Y.; Servi, P.T.; Anbarasan, R. Titania Nanoparticles Synthesized by Sol-gel Technique. Chem. Sci. Trans. 2013, 2, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Racca, L.; Limongi, T.; Vighetto, V.; Dumontel, B.; Ancona, A.; Canta, M.; Canavese, G.; Garino, N.; Cauda, V. Zinc Oxide nanocrystals and high-energy shock waves: A new synergy for the treatment. Front. Bioeng. Biotecnol. 2020, 8, 577. [Google Scholar] [CrossRef]









| a-TiO2-NH2//DOPC-DOTAP 1:5 (m/m) | a-TiO2-NH2//DOPC-DOTAP 1.5:5 (m/m) | |
|---|---|---|
| Colocalization (%) | 88.54 ± 13.75 | 75.0 ± 35.6 |
| US Output Power (W/cm2) | a-TiO2 NPs (Daylight Conditions) [-OH] M | a-TiO2-NH2 NPs (Daylight Conditions) [-OH] M | a-TiO2-NH2 NPs (Dark Conditions) [-OH] M | a-TiO2-NH2//DOPC-DOTAP NPs (Daylight Conditions) [-OH] M |
|---|---|---|---|---|
| 0 | - | 0 | - | - |
| 0.3 | 0 | 0 | - | 0 |
| 0.6 | 0 | 0 | - | 0 |
| 0.9 | 1.81 × 10−5 | 3.297 × 10−5 | 1.73 × 10−6 | 0 |
| 1.2 | 1.54 × 10−5 | 3.295 × 10−5 | 1.98 × 10−5 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, J.C.; Laurenti, M.; Vighetto, V.; Pereira, L.C.J.; Waerenborgh, J.C.; Gonçalves, M.C.; Cauda, V. Biomimetic Amorphous Titania Nanoparticles as Ultrasound Responding Agents to Improve Cavitation and ROS Production for Sonodynamic Therapy. Appl. Sci. 2020, 10, 8479. https://doi.org/10.3390/app10238479
Matos JC, Laurenti M, Vighetto V, Pereira LCJ, Waerenborgh JC, Gonçalves MC, Cauda V. Biomimetic Amorphous Titania Nanoparticles as Ultrasound Responding Agents to Improve Cavitation and ROS Production for Sonodynamic Therapy. Applied Sciences. 2020; 10(23):8479. https://doi.org/10.3390/app10238479
Chicago/Turabian StyleMatos, Joana C., Marco Laurenti, Veronica Vighetto, Laura C. J. Pereira, João Carlos Waerenborgh, M. Clara Gonçalves, and Valentina Cauda. 2020. "Biomimetic Amorphous Titania Nanoparticles as Ultrasound Responding Agents to Improve Cavitation and ROS Production for Sonodynamic Therapy" Applied Sciences 10, no. 23: 8479. https://doi.org/10.3390/app10238479