High Performance Zinc Oxide Nanorod-Doped Ion Imprinted Polypyrrole for the Selective Electrosensing of Mercury II Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments and Characterization
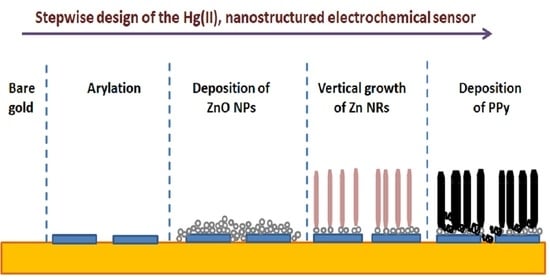
2.3. Surface Functionalization with Diazonium Salt
2.4. Synthesis, Deposition and Vertical Growth of ZnO
2.4.1. Nanoparticle Synthesis
2.4.2. Deposition of ZnO Nanoparticles on Aryl-Modified Gold Electrodes
2.4.3. Vertical Growth of ZnO Nanorods
2.5. Preparation of Au-IIP, Au-ZnO-IIP and Au-ZnO-NIP
2.6. Choice and Role of the Chelating Agent
2.7. Electrochemical Measurements
3. Results and Discussion
3.1. Surface Functionalization by 4-Carboxybenzenediazonium Tetrafluoroborate and Quasi-Vertical Growth of ZnO
3.2. Preparation of IIP and NIP-Based Electrodes
3.3. Surface Analysis by XPS
3.4. Topographical Characterization
3.5. Extraction Time in EDTA and Incubation Time in Mercury Solutions
3.6. Electrochemical Sensing of Mercury
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pacyna, E.G.; Pacyna, J.M. Global Emission of Mercury from Anthropogenic Sources in 1995. Water Air Soil Pollut. 2002, 137, 149–165. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Ayensu, W.K.; Ninashvili, N.; Sutton, D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. Int. J. 2003, 18, 149–175. [Google Scholar] [CrossRef]
- Flanders, J.R.; Long, G.; Reese, B.; Grosso, N.R.; Clements, W.; Stahl, R.G. Assessment of potential mercury toxicity to native invertebrates in a high-gradient stream. Integr. Environ. Assess. Manag. 2019, 15, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Sall, M.L.; Fall, B.; Diédhiou, I.; Dièye, E.H.; Lo, M.; Diaw, A.K.D.; Gningue-Sall, D.; Raouafi, N.; Fall, M. Toxicity and Electrochemical Detection of Lead, Cadmium and Nitrite Ions by Organic Conducting Polymers: A Review. Chem. Afr. 2020. [Google Scholar] [CrossRef]
- Hande, P.E.; Samui, A.B.; Kulkarni, P.S. Selective nanomolar detection of mercury using coumarin based fluorescent Hg (II)—Ion imprinted polymer. Sens. Actuators B Chem. 2017, 246, 597–605. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Wu, T.; Chen, J.; Fu, C.; Zhang, L.; Feng, X.; Fu, X.; Tang, L.; Wang, Z.; et al. Atmospheric mercury emissions from two pre-calciner cement plants in Southwest China. Atmos. Environ. 2019, 199, 177–188. [Google Scholar] [CrossRef]
- Green, C.S.; Lewis, P.J.; Wozniak, J.R.; Drevnick, P.E.; Thies, M.L. A comparison of factors affecting the small-scale distribution of mercury from artisanal small-scale gold mining in a Zimbabwean stream system. Sci. Total Environ. 2019, 647, 400–410. [Google Scholar] [CrossRef]
- Balasundaram, K.; Sharma, M. Technology for mercury removal from flue gas of coal based thermal power plants: A comprehensive review. Crit. Rev. Environ. Sci. Technol. 2019, 48, 1–37. [Google Scholar] [CrossRef]
- D’ltri, P.A.; D’ltri, F.M. Mercury contamination: A human tragedy. Environ. Manag. 1978, 2, 3–16. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Cariccio, V.L.; Samà, A.; Bramanti, P.; Mazzon, E. Mercury Involvement in Neuronal Damage and in Neurodegenerative Diseases. Biol. Trace Elem. Res. 2019, 187, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Guo, Z.; Zeng, G.; Tang, L. Fluorescent and colorimetric sensors for environmental mercury detection. Analyst 2015, 140, 5400–5443. [Google Scholar] [CrossRef] [PubMed]
- Fezai, F.; Gros, P.; Meireles, M.; Evrard, D. New Electrochemical Sensor for Hg(II) Trace Detection in Natural Waters: Electrode Functionalization with Gold Nanoparticles and Diazonium Salts. Meet. Abstr. 2019, MA2019-01, 2010. [Google Scholar]
- Piletsky, S.A.; Turner, A.P.F. Electrochemical Sensors Based on Molecularly Imprinted Polymers. Electroanalysis 2002, 14, 317–323. [Google Scholar] [CrossRef]
- Salmi, Z.; Benzarti, K.; Chehimi, M.M. Diazonium Cation-Exchanged Clay: An Efficient, Unfrequented Route for Making Clay/Polymer Nanocomposites. Langmuir 2013, 29, 13323–13328. [Google Scholar] [CrossRef] [PubMed]
- Msaadi, R.; Ammar, S.; Chehimi, M.M.; Yagci, Y. Diazonium-based ion-imprinted polymer/clay nanocomposite for the selective extraction of lead (II) ions in aqueous media. Eur. Polym. J. 2017, 89, 367–380. [Google Scholar] [CrossRef]
- Lo, M.; Ktari, N.; Gningue-Sall, D.; Madani, A.; Aaron, S.E.; Aaron, J.-J.; Mekhalif, Z.; Delhalle, J.; Chehimi, M.M. Polypyrrole: A reactive and functional conductive polymer for the selective electrochemical detection of heavy metals in water. Emergent Mater. 2020, 1–25. [Google Scholar] [CrossRef]
- Ktari, N.; Fourati, N.; Zerrouki, C.; Ruan, M.; Seydou, M.; Barbaut, F.; Nal, F.; Yaakoubi, N.; Chehimi, M.M.; Kalfat, R. Design of a polypyrrole MIP-SAW sensor for selective detection of flumequine in aqueous media. Correlation between experimental results and DFT calculations. RSC Adv. 2015, 5, 88666–88674. [Google Scholar] [CrossRef]
- Bahrami, A.; Besharati-Seidani, A.; Abbaspour, A.; Shamsipur, M. A highly selective voltammetric sensor for nanomolar detection of mercury ions using a carbon ionic liquid paste electrode impregnated with novel ion imprinted polymeric nanobeads. Mater. Sci. Eng. C 2015, 48, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-C.; Fan, H.-T.; Zhang, Y.; Chen, M.-X.; Yu, Z.-Y.; Cao, X.-Q.; Sun, T. Cd(II)-imprinted polymer sorbents prepared by combination of surface imprinting technique with hydrothermal assisted sol–gel process for selective removal of cadmium(II) from aqueous solution. Chem. Eng. J. 2011, 171, 703–710. [Google Scholar] [CrossRef]
- Karrat, A.; Lamaoui, A.; Amine, A.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. Applications of Chitosan in Molecularly and Ion Imprinted Polymers. Chem. Afr. 2020. [Google Scholar] [CrossRef]
- Fayazi, M.; Ghanei-Motlagh, M.; Taher, M.A.; Ghanei-Motlagh, R.; Salavati, M.R. Synthesis and application of a novel nanostructured ion-imprinted polymer for the preconcentration and determination of thallium(I) ions in water samples. J. Hazard. Mater. 2016, 309, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.R.; Razmpour, S. Synthesis, characterization and application of ion imprinted polymeric nanobeads for highly selective preconcentration and spectrophotometric determination of Ni2+ ion in water samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ashkenani, H.; Taher, M.A. Use of ionic liquid in simultaneous microextraction procedure for determination of gold and silver by ETAAS. Microchem. J. 2012, 103, 185–190. [Google Scholar] [CrossRef]
- Fu, J.; Chen, L.; Li, J.; Zhang, Z. Current status and challenges of ion imprinting. J. Mater. Chem. A 2015, 3, 13598–13627. [Google Scholar] [CrossRef]
- García-Otero, N.; Teijeiro-Valiño, C.; Otero-Romaní, J.; Peña-Vázquez, E.; Moreda-Piñeiro, A.; Moreda-Piñeiro, A. On-line ionic imprinted polymer selective solid-phase extraction of nickel and lead from seawater and their determination by inductively coupled plasma-optical emission spectrometry. Anal. Bioanal. Chem. 2009, 395, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Liu, L.; Deng, F.; Luo, S. Novel ion-imprinted polymer using crown ether as a functional monomer for selective removal of Pb(II) ions in real environmental water samples. J. Mater. Chem. A 2013, 1, 8280–8286. [Google Scholar] [CrossRef]
- Gawin, G.; Konefał, J.; Trzewik, B.; Walas, B.; Tobiasz, A.; Mrowiec, H.; Witek, E. Preparation of a new Cd(II)-imprinted polymer and its application to determination of cadmium(II) via flow-injection-flame atomic absorption spectrometry. Talanta 2010, 80, 1305–1310. [Google Scholar] [CrossRef]
- Abu-Dalo, M.A.; Al-Rawashdeh, N.A.F.; Al-Mheidat, I.R.; Nassory, N.S. Preparation and evaluation of new uranyl imprinted polymer electrode sensor for uranyl ion based on uranyl–carboxybezotriazole complex in pvc matrix membrane. Sens. Actuators B Chem. 2016, 227, 336–345. [Google Scholar] [CrossRef]
- Güney, S.; Güney, O. A novel electrochemical sensor for selective determination of uranyl ion based on imprinted polymer sol–gel modified carbon paste electrode. Sens. Actuators B Chem. 2016, 231, 45–53. [Google Scholar] [CrossRef]
- Alizadeh, T.; Ganjali, M.R.; Zare, M. Application of an Hg2+ selective imprinted polymer as a new modifying agent for the preparation of a novel highly selective and sensitive electrochemical sensor for the determination of ultratrace mercury ions. Anal. Chim. Acta 2011, 689, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.R.; Roushani, M.; Shamsipur, M. Development of a highly selective voltammetric sensor for nanomolar detection of mercury ions using glassy carbon electrode modified with a novel ion imprinted polymeric nanobeads and multi-wall carbon nanotubes. J. Electroanal. Chem. 2013, 693, 16–22. [Google Scholar] [CrossRef]
- Fu, X.-C.; Wu, J.; Nie, L.; Xie, C.-G.; Liu, J.-H.; Huang, X.-J. Electropolymerized surface ion imprinting films on a gold nanoparticles/single-wall carbon nanotube nanohybrids modified glassy carbon electrode for electrochemical detection of trace mercury(II) in water. Anal. Chim. Acta 2012, 720, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-C.; Chen, X.; Guo, Z.; Xie, C.-G.; Kong, L.-T.; Liu, J.-H.; Huang, X.-J. Stripping voltammetric detection of mercury(II) based on a surface ion imprinting strategy in electropolymerized microporous poly(2-mercaptobenzothiazole) films modified glassy carbon electrode. Anal. Chim. Acta 2011, 685, 21–28. [Google Scholar] [CrossRef]
- Li, M.; Gou, H.; Al-Ogaidi, I.; Wu, N. Nanostructured Sensors for Detection of Heavy Metals: A Review. ACS Sustain. Chem. Eng. 2013, 1, 713–723. [Google Scholar] [CrossRef]
- Ait-Touchente, Z.; Falah, S.; Scavetta, E.; Chehimi, M.M.; Touzani, R.; Tonelli, D.; Taleb, A. Different Electrochemical Sensor Designs Based on Diazonium Salts and Gold Nanoparticles for Pico Molar Detection of Metals. Molecules 2020, 25, 3903. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Baratto, C.; Faglia, G.; Sberveglieri, G. Nanostructured ZnO chemical gas sensors. Ceram. Int. 2015, 41, 14239–14244. [Google Scholar] [CrossRef]
- Litton, C.W.; Collins, T.C.; Reynolds, D.C. Zinc Oxide Materials for Electronic and Optoelectronic Device Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-1-119-99121-2. [Google Scholar]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef] [Green Version]
- Luz, G.; Hidalgo, P.; Hui, W.; Nogeuira, P.; Brasil, L. Assembly and Characterization of ZnO nanoparticles for Gratzels Solar Cell. Adv. Mater. Lett. 2018, 9, 284–289. [Google Scholar] [CrossRef]
- Meng, P.; Zhao, X.; Yang, X.; Wu, J.; Xie, Q.; He, J.; Hu, J.; He, J. Breakdown phenomenon of ZnO varistors caused by non-uniform distribution of internal pores. J. Eur. Ceram. Soc. 2019, 39, 4824–4830. [Google Scholar] [CrossRef]
- Jiang, M.; Mao, W.; Zhou, X.; Kan, C.; Shi, D. Wavelength-tunable waveguide emissions from electrically driven single ZnO/ZnO: Ga superlattice microwires. ACS Appl. Mater. Interfaces 2019, 11, 11800–11811. [Google Scholar] [CrossRef]
- Ding, M.; Guo, Z.; Zhou, L.; Fang, X.; Zhang, L.; Zeng, L.; Xie, L.; Zhao, H. One-Dimensional Zinc Oxide Nanomaterials for Application in High-Performance Advanced Optoelectronic Devices. Crystals 2018, 8, 223. [Google Scholar] [CrossRef] [Green Version]
- Wei, A.; Pan, L.; Huang, W. Recent progress in the ZnO nanostructure-based sensors. Mater. Sci. Eng. B 2011, 176, 1409–1421. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Wang, L.; Yang, T.; Guo, X.; Wu, S.; Wang, S. 3D hierarchically porous ZnO structures and their functionalization by Au nanoparticles for gas sensors. J. Mater. Chem. 2010, 21, 349–356. [Google Scholar] [CrossRef]
- Hjiri, M.; El Mir, L.; Leonardi, S.G.; Pistone, A.; Mavilia, L.; Neri, G. Al-doped ZnO for highly sensitive CO gas sensors. Sens. Actuators B Chem. 2014, 196, 413–420. [Google Scholar] [CrossRef]
- Mekki, A.; Ait-Touchente, Z.; Samanta, S.; Singh, A.; Mahmoud, R.; Chehimi, M.M.; Aswal, D.K. Polyaniline-Wrapped ZnO Nanorod Composite Films on Diazonium-Modified Flexible Plastic Substrates. Macromol. Chem. Phys. 2016, 217, 1136–1148. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, L.; Guo, J.; Chen, Q.; Ma, J. ZnO enhanced NiO-based gas sensors towards ethanol. Mater. Res. Bull. 2017, 90, 170–174. [Google Scholar] [CrossRef]
- Navale, Y.H.; Navale, S.T.; Ramgir, N.S.; Stadler, F.J.; Gupta, S.K.; Aswal, D.K.; Patil, V.B. Zinc oxide hierarchical nanostructures as potential NO2 sensors. Sens. Actuators B Chem. 2017, 251, 551–563. [Google Scholar] [CrossRef]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens. Bioelectron. 2019, 141, 111417. [Google Scholar] [CrossRef]
- Wu, J.-J.; Liu, S.-C. Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv. Mater. 2002, 14, 215–218. [Google Scholar] [CrossRef]
- Ashour, A.; Kaid, M.A.; El-Sayed, N.Z.; Ibrahim, A.A. Physical properties of ZnO thin films deposited by spray pyrolysis technique. Appl. Surf. Sci. 2006, 252, 7844–7848. [Google Scholar] [CrossRef]
- Banerjee, A.N.; Ghosh, C.K.; Chattopadhyay, K.K.; Minoura, H.; Sarkar, A.K.; Akiba, A.; Kamiya, A.; Endo, T. Low-temperature deposition of ZnO thin films on PET and glass substrates by DC-sputtering technique. Thin Solid Film. 2006, 496, 112–116. [Google Scholar] [CrossRef]
- Sun, X.W.; Kwok, H.S. Optical properties of epitaxially grown zinc oxide films on sapphire by pulsed laser deposition. J. Appl. Phys. 1999, 86, 408–411. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Kim, B.-S.; Lee, B.-T. Photoluminescence dependence of ZnO films grown on Si (100) by radio-frequency magnetron sputtering on the growth ambient. Appl. Phys. Lett. 2003, 82, 2625–2627. [Google Scholar] [CrossRef]
- Jin, B.J.; Im, S.; Lee, S.Y. Violet and UV luminescence emitted from ZnO thin films grown on sapphire by pulsed laser deposition. Thin Solid Film. 2000, 366, 107–110. [Google Scholar] [CrossRef]
- Yi, J.B.; Pan, H.; Lin, J.Y.; Ding, J.; Feng, Y.P.; Thongmee, S.; Liu, T.; Gong, H.; Wang, L. Ferromagnetism in ZnO Nanowires Derived from Electro-deposition on AAO Template and Subsequent Oxidation. Adv. Mater. 2008, 20, 1170–1174. [Google Scholar] [CrossRef]
- Thongsuriwong, K.; Amornpitoksuk, P.; Suwanboon, S. Structure, morphology, photocatalytic and antibacterial activities of ZnO thin films prepared by sol–gel dip-coating method. Adv. Powder Technol. 2013, 24, 275–280. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Goldberger, J.; Kim, F.; Johnson, J.C.; Zhang, Y.; Saykally, R.J.; Yang, P. Low-temperature wafer-scale production of ZnO nanowire arrays. Angew. Chem. 2003, 115, 3139–3142. [Google Scholar] [CrossRef]
- Pacholski, C.; Kornowski, A.; Weller, H. Self-Assembly of ZnO: From Nanodots to Nanorods. Angew. Chem. Int. Ed. 2002, 41, 1188–1191. [Google Scholar] [CrossRef]
- Chander, R.; Raychaudhuri, A.K. Growth of aligned arrays of ZnO nanorods by low temperature solution method on Si surface. J. Mater. Sci. 2006, 41, 3623–3630. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [Green Version]
- Mazouz, Z.; Touchente, Z.A.; Laradi, H.; Fourati, N.; Yaakoubi, N.; Touzani, R.; Chehimi, M.M.; Kalfat, R.; Othmane, A.; Zerrouki, C. Design of Novel Electrochemical Sensors for the Selective Detection of Glyphosate. In Proceedings of the Eurosensors 2017, Paris, France, 3–6 September 2017; Volume 1, p. 483. [Google Scholar] [CrossRef] [Green Version]
- Ait-Touchente, Z.; Sakhraoui, H.E.E.Y.; Fourati, N.; Zerrouki, C.; Maouche, N.; Touzani, R.; Yaakoubi, N.; Chehimi, M.M. Zinc Oxide Nanorods Wrapped with Ion-Imprinted Polypyrrole Polymer for Picomolar Selective and Electrochemical Detection of Mercury II Ions. Proceedings 2018, 2, 1004. [Google Scholar] [CrossRef] [Green Version]
- Lo, M.; Pires, R.; Diaw, K.; Gningue-Sall, D.; Oturan, M.A.; Aaron, J.-J.; Chehimi, M.M. Diazonium salts: Versatile molecular glues for sticking conductive polymers to flexible electrodes. Surfaces 2018, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Adenier, A.; Bernard, M.-C.; Chehimi, M.M.; Cabet-Deliry, E.; Desbat, B.; Fagebaume, O.; Pinson, J.; Podvorica, F. Covalent Modification of Iron Surfaces by Electrochemical Reduction of Aryldiazonium Salts. J. Am. Chem. Soc. 2001, 123, 4541–4549. [Google Scholar] [CrossRef] [PubMed]
- Hasanpoor, M.; Aliofkhazraei, M.; Delavari, H. Microwave-assisted synthesis of zinc oxide nanoparticles. Procedia Mater. Sci. 2015, 11, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Vasjari, M.; Shirshov, Y.M.; Samoylov, A.V.; Mirsky, V.M. SPR investigation of mercury reduction and oxidation on thin gold electrodes. J. Electroanal. Chem. 2007, 605, 73–76. [Google Scholar] [CrossRef]
- Manceau, A.; Nagy, L.K. Relationships between Hg(ii)–S bond distance and Hg( ii ) coordination in thiolates. Dalton Trans. 2008, 11, 1421–1425. [Google Scholar] [CrossRef]
- Berthon, G. Critical evaluation of the stability constants of metal complexes of amino acids with polar side chains (Technical Report). Pure Appl. Chem. 1995, 67, 1117–1240. [Google Scholar] [CrossRef]
- Sakhraoui, H.E.E.Y.; Mazouz, Z.; Attia, G.; Fourati, N.; Zerrouki, C.; Maouche, N.; Othmane, A.; Yaakoubi, N.; Kalfat, R.; Madani, A.; et al. Design of L-Cysteine and Acrylic Acid Imprinted Polypyrrole Sensors for Picomolar Detection of Lead Ions in Simple and Real Media. IEEE Sens. J. 2019, 20, 4147–4155. [Google Scholar] [CrossRef]
- Ding, N.; Zhao, H.; Peng, W.; He, Y.; Zhou, Y.; Yuan, L.; Zhang, Y. A simple colorimetric sensor based on anti-aggregation of gold nanoparticles for Hg2+ detection. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 161–167. [Google Scholar] [CrossRef]
- Mah, V.; Jalilehvand, F. Mercury (II) complex formation with glutathione in alkaline aqueous solution. JBIC J. Biol. Inorg. Chem. 2008, 13, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Ballav, N.; Das, R.; Giri, S.; Muliwa, A.M.; Pillay, K.; Maity, A. L-cysteine doped polypyrrole (PPy@ L-Cyst): A super adsorbent for the rapid removal of Hg+ 2 and efficient catalytic activity of the spent adsorbent for reuse. Chem. Eng. J. 2018, 345, 621–630. [Google Scholar] [CrossRef]
- Claude, B. Intérêt des Polymères à Empreintes Moléculaires pour la Préparation d’Echantillons par Extraction Solide-Liquide. Application aux Triterpènes dans les Plantes et aux Dopants Dans Les Urines; Université D’Orléans: Orléans, France, 2007; Available online: https://tel.archives-ouvertes.fr/tel-00148669/ (accessed on 5 September 2020).
- Watson, C.M.; Dwyer, D.J.; Andle, J.C.; Bruce, A.E.; Bruce, M.R.M. Stripping Analyses of Mercury Using Gold Electrodes: Irreversible Adsorption of Mercury. Anal. Chem. 1999, 71, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Ordeig, O.; Banks, C.E.; del Campo, J.; Muñoz, F.X.; Compton, R.G. Trace Detection of Mercury(II) Using Gold Ultra-Microelectrode Arrays. Electroanalysis 2006, 18, 573–578. [Google Scholar] [CrossRef]
- Teng, Z.; Lv, H.; Wang, L.; Liu, L.; Wang, C.; Wang, G. Voltammetric Sensor Modified by EDTA-immobilized Graphene-like Carbon Nitride Nanosheets: Preparation, Characterization and Selective Determination of Ultra-Trace Pb (II) in Water Samples. Electrochim. Acta 2016, 212, 722–733. [Google Scholar] [CrossRef]
- Repo, E.; Malinen, L.; Koivula, R.; Harjula, R.; Sillanpää, M. Capture of Co(II) from its aqueous EDTA-chelate by DTPA-modified silica gel and chitosan. J. Hazard. Mater. 2011, 187, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Hinck, M.L.; Ferguson, J.; Puhaakka, J. Resistance of EDTA and DTPA to aerobic biodegradation. Water Sci. Technol. 1997, 35, 25–31. [Google Scholar] [CrossRef]
- Toh, H.S.; Batchelor-McAuley, C.; Tschulik, K.; Damm, C.; Compton, R.G. A proof-of-concept—Using pre-created nucleation centres to improve the limit of detection in anodic stripping voltammetry. Sens. Actuators B Chem. 2014, 193, 315–319. [Google Scholar] [CrossRef]
- Attia, G.; Rahali, S.; Teka, S.; Fourati, N.; Zerrouki, C.; Seydou, M.; Chehimi, S.; Hayouni, S.; Mbakidi, J.-P.; Bouquillon, S.; et al. Anthracene based surface acoustic wave sensors for picomolar detection of lead ions. Correlation between experimental results and DFT calculations. Sens. Actuators B Chem. 2018, 276, 349–355. [Google Scholar] [CrossRef]
- Mazouz, Z.; Rahali, S.; Fourati, N.; Zerrouki, C.; Aloui, N.; Seydou, M.; Yaakoubi, N.; Chehimi, M.M.; Othmane, A.; Kalfat, R. Highly Selective Polypyrrole MIP-Based Gravimetric and Electrochemical Sensors for Picomolar Detection of Glyphosate. Sensors 2017, 17, 2586. [Google Scholar] [CrossRef] [Green Version]
- Roushani, M.; Saedi, Z.; Hamdi, F.; Dizajdizi, B.Z. Preparation an electrochemical sensor for detection of manganese (II) ions using glassy carbon electrode modified with multi walled carbon nanotube-chitosan-ionic liquid nanocomposite decorated with ion imprinted polymer. J. Electroanal. Chem. 2017, 804, 1–6. [Google Scholar] [CrossRef]
- Alizadeh, T.; Rafiei, F.; Hamidi, N.; Ganjali, M.R. A new electrochemical sensing platform for Cr (III) determination based on nano-structured Cr (III)-imprinted polymer-modified carbon composite electrode. Electrochim. Acta 2017, 247, 812–819. [Google Scholar] [CrossRef]
- Alizadeh, T.; Hamidi, N.; Ganjali, M.R.; Rafiei, F. An extraordinarily sensitive voltammetric sensor with picomolar detection limit for Pb2+ determination based on carbon paste electrode impregnated with nano-sized imprinted polymer and multi-walled carbon nanotubes. J. Environ. Chem. Eng. 2017, 5, 4327–4336. [Google Scholar] [CrossRef]
- Lo, M.; Seydou, M.; Bensghaïer, A.; Pires, R.; Gningue-Sall, D.; Aaron, J.-J.; Mekhalif, Z.; Delhalle, J.; Chehimi, M.M. Polypyrrole-Wrapped Carbon Nanotube Composite Films Coated on Diazonium-Modified Flexible ITO Sheets for the Electroanalysis of Heavy Metal Ions. Sensors 2020, 20, 580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthika, A.; Ramasamy Raja, V.; Karuppasamy, P.; Suganthi, A.; Rajarajan, M. Electrochemical behaviour and voltammetric determination of mercury (II) ion in cupric oxide/poly vinyl alcohol nanocomposite modified glassy carbon electrode. Microchem. J. 2019, 145, 737–744. [Google Scholar] [CrossRef]
- Shirzadmehr, A.; Afkhami, A.; Madrakian, T. A new nano-composite potentiometric sensor containing an Hg2+-ion imprinted polymer for the trace determination of mercury ions in different matrices. J. Mol. Liq. 2015, 204, 227–235. [Google Scholar] [CrossRef]
- Armas, M.A.; María-Hormigos, R.; Cantalapiedra, A.; Gismera, M.J.; Sevilla, M.T.; Procopio, J.R. Multiparametric optimization of a new high-sensitive and disposable mercury (II) electrochemical sensor. Anal. Chim. Acta 2016, 904, 76–82. [Google Scholar] [CrossRef]
- Shah, A.; Sultan, S.; Zahid, A.; Aftab, S.; Nisar, J.; Nayab, S.; Qureshi, R.; Khan, G.S.; Hussain, H.; Ozkan, S.A. Highly sensitive and selective electrochemical sensor for the trace level detection of mercury and cadmium. Electrochim. Acta 2017, 258, 1397–1403. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Rahmani, A.R.; Shokoohi, R.; Farmany, A.; Khazaei, M. A Highly Sensitive and Selective Electrochemical Mercury (II) Sensor Based on Nanoparticles of Hg (II)-imprinted Polymer and Graphitic Carbon Nitride (gC 3N4). Int. J. Electrochem. Sci. 2019, 14, 6420–6430. [Google Scholar] [CrossRef]
- Devi, N.R.; Sasidharan, M.; Sundramoorthy, A.K. Gold Nanoparticles-Thiol-Functionalized Reduced Graphene Oxide Coated Electrochemical Sensor System for Selective Detection of Mercury Ion. J. Electrochem. Soc. 2018, 165, B3046–B3053. [Google Scholar] [CrossRef]
- Sánchez-Calvo, A.; Fernández-Abedul, M.T.; Blanco-López, M.C.; Costa-García, A. Paper-based electrochemical transducer modified with nanomaterials for mercury determination in environmental waters. Sens. Actuators B Chem. 2019, 290, 87–92. [Google Scholar] [CrossRef]
- Vu, T.D.; Khac Duy, P.; Bui, H.T.; Han, S.-H.; Chung, H. Reduced graphene oxide–Nickel sulfide (NiS) composited on mechanical pencil lead as a versatile and cost-effective sensor for electrochemical measurements of bisphenol A and mercury (II). Sens. Actuators B Chem. 2019, 281, 320–325. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Tamizhdurai, P.; Ramesh, A.; Chiu, T.-W.; Mangesh, V.L.; Veerarajan, S.; Shanthi, K. Platinum incorporated mordenite zeolite modified glassy carbon electrode used for selective electrochemical detection of mercury ions. Microporous Mesoporous Mater. 2020, 292, 109770. [Google Scholar] [CrossRef]
- Wang, W.; Bao, N.; Yuan, W.; Si, N.; Bai, H.; Li, H.; Zhang, Q. Simultaneous determination of lead, arsenic, and mercury in cosmetics using a plastic based disposable electrochemical sensor. Microchem. J. 2019, 148, 240–247. [Google Scholar] [CrossRef]
- Li, Y.; Xie, J.-F.; Chang, C.-C.; Wang, C.-M.; Tu, H.-L. Highly Sensitive Detection of Mercury Ion Using Zincophosphite Framework Nanoparticle-Polyaniline Composites. ACS Appl. Nano Mater. 2020. [Google Scholar] [CrossRef]















| Samples | Au | C | O | N | Zn | Hg |
|---|---|---|---|---|---|---|
| Bare gold electrode | 41.6 | 42.2 | 16.2 | - | - | - |
| Au/diazo-COOH | 35.8 | 45.9 | 14.3 | 3.97 | - | - |
| Au-diazo-ZnO NPs | - | 60.6 | 28.3 | 0.43 | 10.7 | - |
| Au-diazo-ZnO NRs | 0.87 | 32.3 | 38.5 | 0.97 | 27.4 | - |
| ZnO-IIP | 0.07 | 43.0 | 33.2 | 2.38 | 20.7 | 0.57 |
| ZnO-NIP | 1.61 | 43.5 | 33.3 | 2.95 | 18.7 | - |
| Electrode | Analytical Method | Electrolyte | LOD | Ref |
|---|---|---|---|---|
| Glassy carbon electrode GC/SH/AuNPs | Square wave anodic stripping voltammetry (SWASV) | HCl solution | 10 nM | [13] |
| Carbon-paste electrode (CPE) | Differential pulse voltammetry (DPV) | HCl solution | 0.52 nM | [31] |
| Glassy carbon electrode | Difference pulse voltammetry (DPV) | PBS (pH 7.0) | 0.42 nM | [88] |
| Carbon-paste electrode (CPE) | Electrochemical Impedance Spectroscopy (EIS) | Nitrate solution | 1.95 nM | [89] |
| Commercial screen-printed carbon electrode (SPCE) | Differential pulse voltammetry (DPV) | KNO3/HNO3 solution (pH 2.7) | 0.104 nM | [90] |
| Glassy carbon electrode | Square wave anodic stripping voltammetry (SWASV) | K3[Fe(CN)6] solution | 0.1 nM | [91] |
| Carbon paste electrode (CPE) | Square wave anodic stripping voltammetry (SWASV) | HCl solution | 18 pM | [92] |
| Glassy carbon electrode (GCE) | Differential pulse voltammetry (DPV) | PBS (pH 7.0) | 0.2 μM | [93] |
| Carbon nanomaterials/AuNPs | Anodic stripping voltammetry (ASV) | KCl solution | 0.03 µM | [94] |
| Mechanical Pencil Lead- NiS/Reduced Graphene Oxide | Anodic stripping voltammetry | PBS 1 mg/mL | 0.8 nM | [95] |
| Platinum/(D) mordenite decorated modified glassy carbon electrode (GCE) | Cyclic voltammetry (CV) | KCl solution | 3.4 nM | [96] |
| Gold/polyethylene terephthalate (PET) | Differential pulse voltammetry | HNO3/KCl | 2.49 nM | [97] |
| Zincophosphite NPs (NTOU4nano)/polyaniline (PANI) | Differential pulse voltammetry | PBS (pH 7.0) | 3.49.10−11 M | [98] |
| Zinc oxide nanorods grafted on a Gold electrode | Square Wave Voltammetry (SWV) | KCl solution | 1 pM | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait-Touchente, Z.; Sakhraoui, H.E.E.Y.; Fourati, N.; Zerrouki, C.; Maouche, N.; Yaakoubi, N.; Touzani, R.; Chehimi, M.M. High Performance Zinc Oxide Nanorod-Doped Ion Imprinted Polypyrrole for the Selective Electrosensing of Mercury II Ions. Appl. Sci. 2020, 10, 7010. https://doi.org/10.3390/app10197010
Ait-Touchente Z, Sakhraoui HEEY, Fourati N, Zerrouki C, Maouche N, Yaakoubi N, Touzani R, Chehimi MM. High Performance Zinc Oxide Nanorod-Doped Ion Imprinted Polypyrrole for the Selective Electrosensing of Mercury II Ions. Applied Sciences. 2020; 10(19):7010. https://doi.org/10.3390/app10197010
Chicago/Turabian StyleAit-Touchente, Zouhair, Houssem Eddine El Yamine Sakhraoui, Najla Fourati, Chouki Zerrouki, Naima Maouche, Nourdin Yaakoubi, Rachid Touzani, and Mohamed M. Chehimi. 2020. "High Performance Zinc Oxide Nanorod-Doped Ion Imprinted Polypyrrole for the Selective Electrosensing of Mercury II Ions" Applied Sciences 10, no. 19: 7010. https://doi.org/10.3390/app10197010