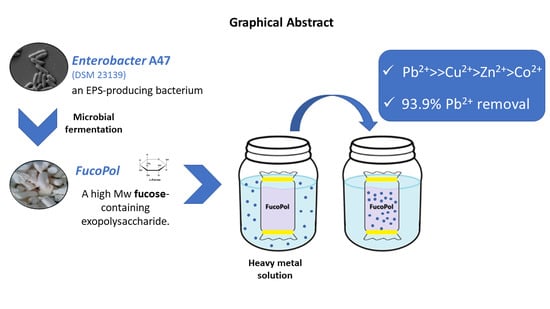
Biosorption of Heavy Metals by the Bacterial Exopolysaccharide FucoPol
Abstract
:1. Introduction
2. Materials and Methods
2.1. FucoPol Production, Purification and Characterization
2.2. Preparation of Solutions
2.3. Metal Biosorption Experiments
2.4. Effect of Dosage, pH and Temperature on Pb2+ Biosorption Ability of FucoPol
2.5. Calculations
3. Results and Discussion
3.1. FucoPol Characterization
3.2. Evaluation of the Metal-Binding Ability of FucoPol
3.3. Effect of Initial Metal Concentration on the Pb2+-Binding Ability of FucoPol
3.4. Effect of FucoPol Dosage on the Pb2+-Binding Ability of FucoPol
3.5. Effect of pH in Pb2+ Removal by FucoPol
3.6. Effect of Temperature in Pb2+ Removal by FucoPol
3.7. Pb2+ Removal by FucoPol: Overall Assessment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abbas, S.H.; Ismail, I.M.; Mostafa, T.M.; Sulaymon, A.H. Biosorption of heavy metals: A review. J. Chem. Sci. Technol. 2014, 3, 74–102. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. ISBN 978-3-7643-8339-8. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Concórdio-Reis, P.; Freitas, F. Environmental Applications: Biopolymer Sorbents for Heavy Metal Removal. In Encyclopedia of Polymer Applications; CRC Press: Boca Raton, FL, USA, 2019; pp. 1069–1086. [Google Scholar]
- Govind, P.; Madhuri, S. Heavy metals causing toxicity in animals and fishes. Res. J. Anim. Vet. Fish. Sci. 2014, 2, 17–23. [Google Scholar]
- Paustenbach, D.J.; Tvermoes, B.E.; Unice, K.M.; Finley, B.L.; Kerger, B.D. A review of the health hazards posed by cobalt. Crit Rev Toxicol. 2013, 43, 316–362. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health 2019, 34, 25–34. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E.; Armijos, R.X.; Weigel, M.M.; Filippelli, G.; Sayegh, M.A. Hepatobiliary-related outcomes in US adults exposed to lead. Environments 2018, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Harari, F.; Sallsten, G.; Christensson, A.; Petkovic, M.; Hedblad, B.; Forsgard, N.; Melander, O.; Nilsson, P.M.; Borné, Y.; Engström, G.; et al. Blood lead levels and decreased kidney function in a population-based cohort. Am. J. Kidney Dis. 2018, 72, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Lanphear, B.P.; Rauch, S.; Auinger, P.; Allen, R.W.; Hornung, R.W. Low-level lead exposure and mortality in US adults: A population-based cohort study. Lancet Public Health 2018, 3, e177–e184. [Google Scholar] [CrossRef]
- Fu, Z.; Wu, F.; Chen, L.; Xu, B.; Feng, C.; Bai, Y.; Liao, H.; Sun, S.; Giesy, J.P.; Guo, W. Copper and zinc, but not other priority toxic metals, pose risks to native aquatic species in a large urban lake in Eastern China. Environ. Pollut. 2016, 219, 1069–1076. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An Overview of Carcinogenic Heavy Metal: Molecular Toxicity Mechanism and Prevention. J. Cancer Prev. 2015, 20, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Caspi, A.; Belsky, D.W.; Broadbent, J.; Harrington, H.; Sugden, K.; Houts, R.M.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood. JAMA 2017, 317, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Gyasi, E. Lead exposure and oxidative stress—A life course approach in US adults. Toxics 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, S.K.; Gaur, J.P. Use of Algae for Removing Heavy Metal Ions from Wastewater: Progress and Prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Biswas, J.K.; Banerjee, A.; Sarkar, B.; Sarkar, D.; Sarkar, S.K.; Rai, M.; Vithanage, M. Exploration of an Extracellular Polymeric Substance from Earthworm Gut Bacterium (Bacillus licheniformis) for Bioflocculation and Heavy Metal Removal Potential. Appl. Sci. 2020, 10, 349. [Google Scholar] [CrossRef] [Green Version]
- Brinza, L.; Dring, M.J.; Gavrilescu, M. Marine micro and macro algal species as biosorbents for heavy metals. Environ. Eng. Manag. J. EEMJ 2007, 6, 237–251. [Google Scholar] [CrossRef]
- Morillo Pérez, J.A.; García-Ribera, R.; Quesada, T.; Aguilera, M.; Ramos-Cormenzana, A.; Monteoliva-Sánchez, M. Biosorption of heavy metals by the exopolysaccharide produced by Paenibacillus jamilae. World J. Microbiol. Biotechnol. 2008, 24, 2699–2704. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Shojaosadati, S.A. Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res. 2003, 37, 4231–4235. [Google Scholar] [CrossRef]
- Lin, J.; Harichund, C. Production and characterization of heavy-metal removing bacterial bioflocculants. Afr. J. Biotechnol. 2012, 11, 9619–9629. [Google Scholar] [CrossRef]
- Fella-Temzi, S.; Yalaoui-Guellal, D.; Rodriguez-Carvajal, M.A.; Belhadi, D.; Madani, K.; Kaci, Y. Removal of lead by exopolysaccharides from Paenibacillus peoriae strainTS7 isolated from rhizosphere of durum wheat. Biocatal. Agric. Biotechnol. 2018, 16, 425–432. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.; Mas Haris, M.R.H. Application of biopolymer composites in arsenic removal from aqueous medium: A review. J. Radiat. Res. Appl. Sci. 2015, 8, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Freitas, F.; Alves, V.D.; Torres, C.A.V.; Cruz, M.; Sousa, I.; Melo, M.J.; Ramos, A.M.; Reis, M.A.M. Fucose-containing exopolysaccharide produced by the newly isolated Enterobacter strain A47 DSM 23139. Carbohydr. Polym. 2011, 83, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Concórdio-Reis, P.; Pereira, C.V.; Batista, M.P.; Sevrin, C.; Grandfils, C.; Marques, A.C.; Fortunato, E.; Gaspar, F.B.; Matias, A.A.; Freitas, F.; et al. Silver nanocomposites based on the bacterial fucose-rich polysaccharide secreted by Enterobacter A47 for wound dressing applications: Synthesis, characterization and in vitro bioactivity. Int. J. Biol. Macromol. 2020, 163, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, B.M.; Freitas, F.; Lima, J.C.; Silva, J.C.; Dionísio, M.; Reis, M.A.M. Demonstration of the cryoprotective properties of the fucose-containing polysaccharide FucoPol. Carbohydr. Polym. 2020, 245, 116500. [Google Scholar] [CrossRef]
- Dhadge, V.L.; Morgado, P.I.; Freitas, F.; Reis, M.A.; Azevedo, A.; Aires-Barros, R.; Roque, A.C.A. An extracellular polymer at the interface of magnetic bioseparations. J. R. Soc. Interface 2014, 11, 20140743. [Google Scholar] [CrossRef]
- Palma, S.I.C.J.; Rodrigues, C.A.V.; Carvalho, A.; Morales, M.d.P.; Freitas, F.; Fernandes, A.R.; Cabral, J.M.S.; Roque, A.C.A. A value-added exopolysaccharide as a coating agent for MRI nanoprobes. Nanoscale 2015, 7, 14272–14283. [Google Scholar] [CrossRef]
- Concórdio-Reis, P.; Pereira, J.R.; Torres, C.A.V.; Sevrin, C.; Grandfils, C.; Freitas, F. Effect of mono- and dipotassium phosphate concentration on extracellular polysaccharide production by the bacterium Enterobacter A47. Process Biochem. 2018, 75, 16–21. [Google Scholar] [CrossRef]
- Maalej, H.; Hmidet, N.; Boisset, C.; Buon, L.; Heyraud, A.; Nasri, M. Optimization of exopolysaccharide production from Pseudomonas stutzeri AS22 and examination of its metal-binding abilities. J. Appl. Microbiol. 2015, 118, 356–367. [Google Scholar] [CrossRef]
- Loaëc, M.; Olier, R.; Guezennec, J. Chelating properties of bacterial exopolysaccharides from deep-sea hydrothermal vents. Carbohydr. Polym. 1998, 35, 65–70. [Google Scholar] [CrossRef]
- Yan, Z.; Xuliang, F.; Zhilong, Y.; Yahong, L.; Weimin, C. Biosorption of Cu (II) on extracellular polymers from Bacillus sp. F19. J. Environ. Sci. 2008, 20, 1288–1293. [Google Scholar]
- Kim, S.-Y.; Kim, J.-H.; Kim, C.-J.; Oh, D.-K. Metal adsorption of the polysaccharide produced from Methylobacterium organophilum. Biotechnol. Lett. 1996, 18, 1161–1164. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Production and Characteristics of a Heavy Metals Removing Bioflocculant Produced by Pseudomonas aeruginosa. Pol. J. Microbiol. 2012, 61, 281–289. [Google Scholar]
- Maalej, H.; Boisset, C.; Hmidet, N.; Buon, L.; Heyraud, A.; Nasri, M. Purification and structural data of a highly substituted exopolysaccharide from Pseudomonas stutzeri AS22. Carbohydr. Polym. 2014, 112, 404–411. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Yang, J.; Ma, F. Pb(II) biosorption by compound bioflocculant: Performance and mechanism. Desalin. Water Treat. 2015, 53, 421–429. [Google Scholar] [CrossRef]
- Can, C.; Jianlong, W. Correlating metal ionic characteristics with biosorption capacity using QSAR model. Chemosphere 2007, 69, 1610–1616. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Torres, C.A.V.; Ferreira, A.R.V.; Freitas, F.; Reis, M.A.M.; Coelhoso, I.; Sousa, I.; Alves, V.D. Rheological studies of the fucose-rich exopolysaccharide FucoPol. Int. J. Biol. Macromol. 2015, 79, 611–617. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- López, A.; Lazaro, N.; Priego, J.M.; Marques, A.M. Effect of pH on the biosorption of nickel and other heavy metals by Pseudomonas fluorescens 4F39. J. Ind. Microbiol. Biotechnol. 2000, 24, 146–151. [Google Scholar] [CrossRef]
- Deng, L.; Su, Y.; Su, H.; Wang, X.; Zhu, X. Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J. Hazard. Mater. 2007, 143, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M.; Kibret, M. Recent Trends in Microbial Biosorption of Heavy Metals: A Review. Biochem. Mol. Biol. 2013, 1, 19–26. [Google Scholar] [CrossRef]
- Subudhi, S.; Batta, N.; Pathak, M.; Bisht, V.; Devi, A.; Lal, B.; Al khulifah, B. Bioflocculant production and biosorption of zinc and lead by a novel bacterial species, Achromobacter sp. TERI-IASST N, isolated from oil refinery waste. Chemosphere 2014, 113, 116–124. [Google Scholar] [CrossRef]
- Mota, R.; Rossi, F.; Andrenelli, L.; Pereira, S.B.; De Philippis, R.; Tamagnini, P. Released polysaccharides (RPS) from Cyanothece sp. CCY 0110 as biosorbent for heavy metals bioremediation: Interactions between metals and RPS binding sites. Appl. Microbiol. Biotechnol. 2016, 100, 7765–7775. [Google Scholar] [CrossRef]
 ) and specific metal uptake (q, ●) (temperature 21 °C, incubation time 24 h).
) and specific metal uptake (q, ●) (temperature 21 °C, incubation time 24 h).
 ) and specific metal uptake (q, ●) (temperature 21 °C, incubation time 24 h).
) and specific metal uptake (q, ●) (temperature 21 °C, incubation time 24 h).
 ) by FucoPol (5 g/L) using different initial Pb2+ concentrations (temperature 21 °C, incubation time 24 h).
) by FucoPol (5 g/L) using different initial Pb2+ concentrations (temperature 21 °C, incubation time 24 h).
 ) by FucoPol (5 g/L) using different initial Pb2+ concentrations (temperature 21 °C, incubation time 24 h).
) by FucoPol (5 g/L) using different initial Pb2+ concentrations (temperature 21 °C, incubation time 24 h).
 ) (pH 4.3, temperature 21 °C, incubation time 24 h).
) (pH 4.3, temperature 21 °C, incubation time 24 h).
 ) (pH 4.3, temperature 21 °C, incubation time 24 h).
) (pH 4.3, temperature 21 °C, incubation time 24 h).


| Organism | Sugar Composition (wt% or Molar Ratio) | Non-Sugar Residues (wt%) | Mw (Da) | Bisorption Capacity (mg/gEPS or %) | References |
|---|---|---|---|---|---|
| Alteromonas macleodii subsp fijiensis | GlcA, Glc, Gal, Man and GalA (2.4:1.6:1.4:1.1:1.0) | Protein (4 %) | n.a. | Pb2+: 316 Zn2+: 75 | [33] |
| Bacillus firmus MS-102 | Glc, Fru, Man, Gal (12.1:5.7:3.1:1.0) Uronic acids (38 %) | Pyr (6.3 %) | n.a. | Pb2+: 1103 or 98.3% Cu2+: 860 or 74.9% Zn2+: 722 or 61.8% | [21] |
| Bacillus sp. F19 | Man, Glc (1.2:1) Uronic acids (37 %) amino sugars (0.5 %) | Protein (16.4%) | n.a. | Cu2+: 89.6 | [34] |
| Enterobacter A47 | Fuc, Gal, Glc and GlcA (2.0:1.9:0.9:0.5) | Protein (10.8%) Ac, Pyr, Succ (12.3%) | 4.4 × 106 | Pb2+: 108.0 or 93.9% Cu2+: 23.5 or 36.1% Zn2+: 20.1 or 35.9% Co2+: 20.3 or 32.7% | This study |
| Methylobacterium organophilum | Gal, Man, Glu (3:2:2) Uronic acids (12.4%) | Protein (6.1%) Pyr (5.1%) Ac (0.6 %) | n.a. | Pb2+: 184.2 Cu2+: 200.3 | [35] |
| Paenibacillus jamilae | Glc, Man, Gal, Fuc, Rha (54.6, 25.6, 12.9, 3.8, 3.1%) Uronic acids (28.3%) Aminosugars (2.8%) | Protein (1.5%) Pyr (8.7%) Acetyls (4.13%) | n.a. | Pb2+: 303.0 Cu2+: 12.3 Zn2+: 7.8 Co2+: 20.5 | [20] |
| Paenibacillus peoriae TS7 | Fru | - | n.a. | Pb2+: 277.5 | [23] |
| Pseudomonas aeruginosa ATCC-10145 | Neutral sugars (30.6%) Uronic acids (2.35%) Aminosugars (0.78%) | Protein (27%) | n.a. | Pb2+: 79.7% Cu2+: 87.4% Zn2+: 80.6% | [36] |
| Pseudomonas stuteri AS22 | Glc, Man, Lactyl rhamnose (1:1.1:0.7) | Lactyl, acetyl and pyruvyl groups | 9.9 × 105 | Pb2+: 215.6 Cu2+: 0.6 Co2+: 1.4 | [32,37] |
| Rhizobium radiobacter F2 and Bacillus sphaeicus F6 | Glc, Man, Rha, Gal (10.0:2.1:1.3:1.0) | - | 4.79 × 105 | Pb2+: 189.3 | [38] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concórdio-Reis, P.; Reis, M.A.M.; Freitas, F. Biosorption of Heavy Metals by the Bacterial Exopolysaccharide FucoPol. Appl. Sci. 2020, 10, 6708. https://doi.org/10.3390/app10196708
Concórdio-Reis P, Reis MAM, Freitas F. Biosorption of Heavy Metals by the Bacterial Exopolysaccharide FucoPol. Applied Sciences. 2020; 10(19):6708. https://doi.org/10.3390/app10196708
Chicago/Turabian StyleConcórdio-Reis, Patrícia, Maria A. M. Reis, and Filomena Freitas. 2020. "Biosorption of Heavy Metals by the Bacterial Exopolysaccharide FucoPol" Applied Sciences 10, no. 19: 6708. https://doi.org/10.3390/app10196708