FTIR Microspectroscopy for the Assessment of Mycoplasmas in HepG2 Cell Culture
Abstract
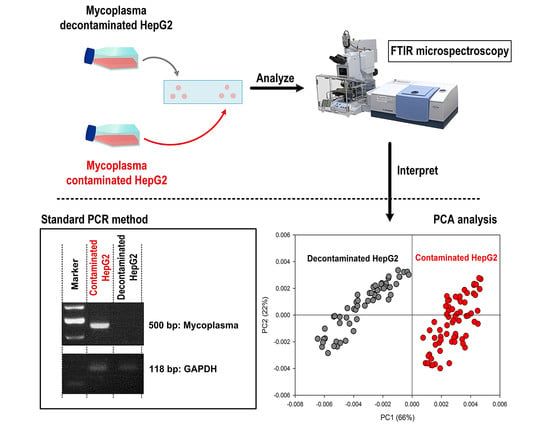
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Cell Lines and Culture
2.3. Antibiotic Treatment of Mycoplasmas Contaminants
2.4. DNA Extraction
2.5. PCR-Based Detection of Mycoplasmas
2.6. FTIR Microspectroscopy
2.7. Statistical Analysis
3. Results and Discussion
3.1. Detection of Mycoplasma by PCR
3.2. Spectral Observation
3.3. PCA and Histogram Analysis
3.4. Spectral Marker Inspection
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rottem, S. Interaction of mycoplasmas with host cells. Physiol. Rev. 2003, 83, 417–432. [Google Scholar] [CrossRef] [Green Version]
- Drexler, H.G.; Uphoff, C.C. Mycoplasma contamination of cell cultures: Incidence, sources, effects, detection, elimination, prevention. Cytotechnology 2002, 39, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Malygina, T.Y.; Mouzykantov, A.A.; Medvedeva, E.S.; Baranova, N.B.; Chernova, O.A.; Chernov, V.M.; Trushin, M.V.; Aminov, R.I. Antimicrobial resistance in mollicutes: Known and newly emerging mechanisms. FEMS Mirobiol. Lett. 2018, 365. [Google Scholar] [CrossRef]
- Chernova, O.A.; Medvedeva, E.S.; Mouzykantov, A.A.; Baranova, N.B.; Chernov, V.M. Mycoplasmas and their antibiotic resistance: The problems and prospects in controlling infections. Acta Nat. 2016, 8, 24–34. [Google Scholar]
- Degeling, M.H.; Maguire, C.A.; Bovenberg, M.S.; Tannous, B.A. Sensitive assay for mycoplasma detection in mammalian cell culture. Anal. Chem. 2012, 84, 4227–4232. [Google Scholar] [PubMed] [Green Version]
- Sahu, R.; Mordechai, S. Fourier transform infrared spectroscopy in cancer detection. Future. Oncol. 2005, 1, 635–647. [Google Scholar] [CrossRef] [Green Version]
- Bellisola, G.; Sorio, C. Infrared spectroscopy and microscopy in cancer research and diagnosis. Am. J. Cancer Res. 2011, 2, 1–21. [Google Scholar]
- Zelig, U.; Kapelushnik, J.; Moreh, R.; Mordechai, S.; Nathan, I. Diagnosis of cell death by means of infrared spectroscopy. Biophys. J. 2009, 97, 2107–2114. [Google Scholar] [CrossRef] [Green Version]
- Sablinskas, V.; Velicka, M.; Pucetaite, M.; Urboniene, V.; Ceponkus, J.; Bandzeviciute, R.; Jankevicius, F.; Sakharova, T.; Bibikova, O.; Steiner, G. In Situ Detection of Cancerous Kidney Tissue by Means of Fiber ATR-FTIR Spectroscopy; SPIE: Bellingham, WA, USA, 2018; Volume 10497. [Google Scholar]
- Theophilou, G.; Lima, K.M.; Martin-Hirsch, P.L.; Stringfellow, H.F.; Martin, F.L. ATR-FTIR spectroscopy coupled with chemometric analysis discriminates normal, borderline and malignant ovarian tissue: Classifying subtypes of human cancer. Analyst 2016, 141, 585–594. [Google Scholar]
- Rottem, S.; Kosower, N.; Kornspan, D. Contamination of tissue cultures by mycoplasmas. Biomed. Tissue Cult. 2012, 35–58. [Google Scholar] [CrossRef] [Green Version]
- Timenetsky, J.; Santos, L.M.; Buzinhani, M.; Mettifogo, E. Detection of multiple mycoplasma infection in cell cultures by PCR. Braz. J. Med. Biol. Res. 2006, 39, 907–914. [Google Scholar] [PubMed] [Green Version]
- Wehbe, K.; Vezzalini, M.; Cinque, G. Detection of mycoplasma in contaminated mammalian cell culture using FTIR microspectroscopy. Anal. Bioanal. Chem. 2018, 410, 3003–3016. [Google Scholar] [PubMed] [Green Version]
- Dreolini, L.; Cullen, M.; Yung, E.; Laird, L.; Webb, J.R.; Nelson, B.H.; Hay, K.A.; Balasundaram, M.; Kekre, N.; Holt, R.A. A rapid and sensitive nucleic acid amplification technique for mycoplasma screening of cell therapy products. Mol. Ther. Method Clin. Dev. 2020, 17, 393–399. [Google Scholar]
- Ali, S.; Mahmood, A. Microbial and viral contamination of animal and stem cell cultures: Common contaminants, detection and elimination. JSRT 2017, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Mourant, J.R.; Yamada, Y.R.; Carpenter, S.; Dominique, L.R.; Freyer, J.P. FTIR Spectroscopy demonstrates biochemical differences in mammalian cell cultures at different growth stages. Biophys J. 2003, 85, 1938–1947. [Google Scholar] [CrossRef] [Green Version]
- Gedye, C.; Cardwell, T.; Dimopoulos, N.; Tan, B.S.; Jackson, H.; Svobodova, S.; Anaka, M.; Behren, A.; Maher, C.; Hofmann, O.; et al. Mycoplasma infection alters cancer stem cell properties in vitro. Stem. Cell. Rev. 2016, 12, 156–161. [Google Scholar]
- Chuerduangphui, J.; Proyrungroj, K.; Pientong, C.; Hinkan, S.; Budkaew, J.; Pimson, C.; Chumworathayi, B.; Hanond, P.; Ekalaksananan, T. Prevalence and anatomical sites of human papillomavirus, Epstein-Barr virus and herpes simplex virus infections in men who have sex with men, Khon Kaen, Thailand. BMC Infect. Dis. 2018, 18, 509. [Google Scholar]
- Junhom, C.; Weerapreeyakul, N.; Tanthanuch, W.; Thumanu, K. FTIR microspectroscopy defines early drug resistant human hepatocellular carcinoma (HepG2) cells. Exp. Cell Res. 2016, 340, 71–80. [Google Scholar] [CrossRef]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Thumanu, K.; Tanthanuch, W. FTIR microspectroscopy discriminates anticancer action on human leukemic cells by extracts of Pinus kesiya; Cratoxylum formosum ssp. pruniflorum and melphalan. Talanta 2012, 93, 371–382. [Google Scholar] [CrossRef]
- Bouyanfif, A.; Liyanage, S.; Hequet, E.; Moustaid-Moussa, N.; Abidi, N. FTIR microspectroscopy reveals fatty acid-induced biochemical changes in C. elegans. Vib. Spectrosc. 2019, 102, 8–15. [Google Scholar] [CrossRef]
- Dreissig, I.; Machill, S.; Salzer, R.; Krafft, C. Quantification of brain lipids by FTIR spectroscopy and partial least squares regression. Spectrochim. Acta A 2009, 71, 2069–2075. [Google Scholar]
- Naumann, A. Fourier transform infrared (FTIR) microscopy and imaging of fungi. In Advanced Microscopy in Mycology; Dahms, T.E.S., Czymmek, K.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 61–88. [Google Scholar]
- Boncheva, M.; Damien, F.; Normand, V. Molecular organization of the lipid matrix in intact Stratum corneum using ATR-FTIR spectroscopy. Biochim. Biophys. Acta 2008, 1778, 1344–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, D.R.; Bambery, K.R.; Puskar, L.; McNaughton, D.; Wood, B.R. Quantification of DNA in simple eukaryotic cells using Fourier transform infrared spectroscopy. J. Biophotonics 2013, 6, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar]
- Maity, D.; Kanti Bain, M.; Bhowmick, B.; Sarkar, J.; Saha, S.; Acharya, K.; Chakraborty, M.; Chattopadhyay, D. In situ synthesis, characterization, and antimicrobial activity of silver nanoparticles using water soluble polymer. J. Appl. Polym. Sci. 2011, 122, 2189–2196. [Google Scholar] [CrossRef]
- Rehman, I.; Movasaghi, Z.; Rehman, S. Vibrational Spectroscopy for Tissue Analysis; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Bioch. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Kumosinski, T.F.; Farrell, H.M. Determination of the global secondary structure of proteins by Fourier transform infrared (FTIR) spectroscopy. Trends Food Sci. Tech. 1993, 4, 169–175. [Google Scholar] [CrossRef]
- Baldassarre, M.; Li, C.; Eremina, N.; Goormaghtigh, E.; Barth, A. Simultaneous fitting of absorption spectra and their second derivatives for an improved analysis of protein infrared spectra. Molecules 2015, 20, 12599–12622. [Google Scholar]
- Zhu, H.; Liu, P.; Du, J.; Wang, J.; Jing, Y.; Zhang, J.; Gu, W.; Wang, W.; Meng, Q. Identification of lysophospholipase protein from Spiroplasma eriocheiris and verification of its function. Microbiology 2017, 163, 175–184. [Google Scholar] [CrossRef]
- Madeira, H.M.F.; Gabriel, J.E. Regulation of gene expression in mycoplasmas: Contribution from mycoplasma hyopneumoniae and mycoplasma synoviae genome sequences. Genet. Mol. Biol. 2007, 30, 277–282. [Google Scholar]
- Hopfe, M.; Deenen, R.; Degrandi, D.; Köhrer, K.; Henrich, B. Host cell responses to persistent mycoplasmas—Different stages in infection of HeLa cells with Mycoplasma hominis. PLoS ONE 2013, 8, e54219. [Google Scholar] [PubMed]
- Hoff, F.W.; Hu, C.W.; Qutub, A.A.; Qiu, Y.; Graver, E.; Hoang, G.; Chauhan, M.; de Bont, E.S.J.M.; Kornblau, S.M. Mycoplasma contamination of leukemic cell lines alters protein expression determined by reverse phase protein arrays. Cytotechnology 2018, 70, 1529–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holman, H.Y.; Martin, M.C.; Blakely, E.A.; Bjornstad, K.; McKinney, W.R. IR spectroscopic characteristics of cell cycle and cell death probed by synchrotron radiation based Fourier transform IR spectromicroscopy. Biopolymers 2000, 57, 329–335. [Google Scholar] [CrossRef]
- Gerlic, M.; Horowitz, J.; Horowitz, S. Mycoplasma fermentans inhibits tumor necrosis factor α-induced apoptosis in the human myelomonocytic U937 cell line. Cell Death Differ. 2004, 11, 1204. [Google Scholar]
- Boydston-White, S.; Gopen, T.; Houser, S.; Bargonetti, J.; Diem, M. Infrared spectroscopy of human tissue. V. Infrared spectroscopic studies of myeloid leukemia (ML-1) cells at different phases of the cell cycle. Biospectroscopy 1999, 5, 219–227. [Google Scholar] [CrossRef]
- Moullan, N.; Mouchiroud, L.; Wang, X.; Ryu, D.; Williams, E.G.; Mottis, A.; Jovaisaite, V.; Frochaux, M.V.; Quiros, P.M.; Deplancke, B.; et al. Tetracyclines disturb mitochondrial function across eukaryotic models: A call for caution in biomedical research. Cell Rep. 2015, 10, 1681–1691. [Google Scholar] [CrossRef] [Green Version]
- Uphoff, C.C.; Drexler, H.G. Comparative PCR analysis for detection of mycoplasma infections in continuous cell lines. Vitro Cell Dev. Biol. Anim. 2002, 38, 79–85. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Kozakai, T.; Morita, H.; Saida, K.; Oka, S.; Masuo, Y. Rapid detection of mycoplasma contamination in cell cultures using sybr green-based real-time polymerase chain reaction. Vitro Cell Dev. Biol. Anim. 2006, 42, 63–69. [Google Scholar]






| Targets | Sequences (5′‒3′) | Product Size |
|---|---|---|
| Mycoplasmas [17] | F: GGCGAATGGGTGAGTAACACG | 500 |
| R: GGATAACGCTTTGCGACCTATG | ||
| GAPDH [18] | F: TCATCAGCAATGCCTCCTGCA | 118 |
| R: TGG GTG GCA GTG ATG GCA |
| Wave Number (cm−1) | Primary Spectral Difference * | PCA Score Plot ** | Functional Group | Vibrational Mode *** | Band Assignment | |
|---|---|---|---|---|---|---|
| PC1 | PC2 | |||||
| 2923 | 🡳 | (–) | (–) | −(CH2)n− | νas(CH2) | Lipid |
| 2852 | 🡳 | (–) | (–) | −(CH2)n− | νs(CH2) | Lipid |
| 1743 | 🡳 | (–) | −CH2−COOR | ν(C=O) | Phospholipid esters | |
| 1712 | 🡳 | C=O | ν(C=O) | Nucleic acid | ||
| 1650 | 🡱 | + | + | O=C−N−H | 80% ν(C=O)/20% ν(CN) | Amide I peptide |
| 1639 | 🡱 | + | ||||
| 1624 | 🡱 | (–) | ||||
| 1552 | 🡳 | O=C−N−H | 60% γ(NH)/ 30%ν(CN)/10% ν(CC) | Amide II peptide | ||
| 1544 | 🡱 | + | + | |||
| 1512 | 🡱 | - | δCH | CH in phenyl ring | ||
| 1467 | 🡳 | + | −(CH2)n− | δCH2 | Lipid | |
| 1380 | 🡳 | C−CH3 | γs(CH3) | Lipid | ||
| 1236 | 🡱 | RO−PO2−−OR | νas(PO2−) | DNA/RNA, phospholipid ester | ||
| 1170 | 🡳 | R−COO−R’ | νas(CO) | Ester | ||
| 1052 | 🡳 | C−O−P | ν (COP) | Phosphate ester | ||
| 968 | 🡳 | PO32− | νas(PO32−) | DNA/RNA ribose | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pocasap, P.; Weerapreeyakul, N.; Junhom, C.; Phiboonchaiyanan, P.P.; Srisayam, M.; Nonpunya, A.; Siriwarin, B.; Khamphio, M.; Nanok, C.; Thumanu, K.; et al. FTIR Microspectroscopy for the Assessment of Mycoplasmas in HepG2 Cell Culture. Appl. Sci. 2020, 10, 3766. https://doi.org/10.3390/app10113766
Pocasap P, Weerapreeyakul N, Junhom C, Phiboonchaiyanan PP, Srisayam M, Nonpunya A, Siriwarin B, Khamphio M, Nanok C, Thumanu K, et al. FTIR Microspectroscopy for the Assessment of Mycoplasmas in HepG2 Cell Culture. Applied Sciences. 2020; 10(11):3766. https://doi.org/10.3390/app10113766
Chicago/Turabian StylePocasap, Piman, Natthida Weerapreeyakul, Cholpajsorn Junhom, Preeyaporn Plaimee Phiboonchaiyanan, Montra Srisayam, Apiyada Nonpunya, Boondaree Siriwarin, Munthipha Khamphio, Chaiyachet Nanok, Kanjana Thumanu, and et al. 2020. "FTIR Microspectroscopy for the Assessment of Mycoplasmas in HepG2 Cell Culture" Applied Sciences 10, no. 11: 3766. https://doi.org/10.3390/app10113766