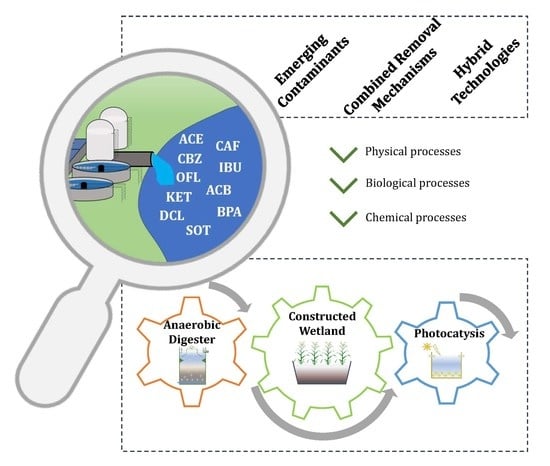
The Potential of Constructed Wetland Systems and Photodegradation Processes for the Removal of Emerging Contaminants—A Review
Abstract
:1. Introduction
2. Methodological Considerations
3. General Characteristics and Physicochemical Properties of Selected EOCs
3.1. General Characteristics of Target EOCs
3.2. Physicochemical Properties of the Selected EOCs
4. Removal of EOCs from Municipal Wastewater: Technologies and Mechanisms
4.1. EOC Removal Technologies for Municipal Wastewater
4.2. EOC Removal by Sorption
4.3. Biodegradation
5. EOC Removal through CW-Based Systems
5.1. Anaerobic Digesters
5.2. Constructed Wetlands
5.2.1. Main Factors Affecting the Removal of EOCs in CWs
5.2.2. Acetaminophen
5.2.3. Ofloxacin
5.2.4. Carbamazepine
5.2.5. Caffeine
5.2.6. Ketoprofen
5.2.7. Ibuprofen
5.2.8. Diclofenac
5.2.9. Clofibric Acid
5.2.10. Bisphenol A
5.2.11. Sotalol
6. Potential of Photodegradation with TiO2 Catalyst as Post-Treatment of CW Effluents
6.1. Combining CWs and TiO2-Based Photocatalysis
6.2. Removal of EOCs from CW Effluents Using TiO2-Based Photocatalysis
7. Overall Comparison and Some Research Gaps
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Saidulu, D.; Gupta, B.; Gupta, A.K.; Ghosal, P.S. A Review on Occurrences, Eco-Toxic Effects, and Remediation of Emerging Contaminants from Wastewater: Special Emphasis on Biological Treatment Based Hybrid Systems. J. Environ. Chem. Eng. 2021, 9, 105282. [Google Scholar] [CrossRef]
- Khasawneh, O.; Palaniandy, P. Occurrence and Removal of Pharmaceuticals in Wastewater Treatment Plants. Process Saf. Environ. Prot. 2021, 150, 532–556. [Google Scholar] [CrossRef]
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically Active Compounds in Aqueous Environment: A Status, Toxicity and Insights of Remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity—A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Huang, R.P.; Liu, Z.H.; Yin, H.; Dang, Z.; Wu, P.X.; Zhu, N.W.; Lin, Z. Bisphenol A Concentrations in Human Urine, Human Intakes across Six Continents, and Annual Trends of Average Intakes in Adult and Child Populations Worldwide: A Thorough Literature Review. Sci. Total Environ. 2018, 626, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions-a Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of Selected Pharmaceuticals, Fragrances and Endocrine Disrupting Compounds in a Membrane Bioreactor and Conventional Wastewater Treatment Plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef]
- Aymerich, I.; Acuña, V.; Barceló, D.; García, M.J.; Petrovic, M.; Poch, M.; Rodriguez-Mozaz, S.; Rodríguez-Roda, I.; Sabater, S.; von Schiller, D.; et al. Attenuation of Pharmaceuticals and Their Transformation Products in a Wastewater Treatment Plant and Its Receiving River Ecosystem. Water Res. 2016, 100, 126–136. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Biological Combination Processes for Efficient Removal of Pharmaceutically Active Compounds from Wastewater: A Review and Future Perspectives. J. Environ. Chem. Eng. 2017, 5, 3590–3603. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Bojanowska-Czajka, A.; Trojanowicz, M. Comparison of Different Advanced Degradation Processes for the Removal of the Pharmaceutical Compounds Diclofenac and Carbamazepine from Liquid Solutions. Environ. Sci. Pollut. Res. 2018, 25, 27704–27723. [Google Scholar] [CrossRef] [PubMed]
- Masi, F.; Rizzo, A.; Regelsberger, M. The Role of Constructed Wetlands in a New Circular Economy, Resource Oriented, and Ecosystem Services Paradigm. J. Environ. Manag. 2018, 216, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, I.; Díaz, M.A.; Crujeiras, B.; García, J.; Soto, M. Solids Hydrolysis and Accumulation in a Hybrid Anaerobic Digester-Constructed Wetlands System. Ecol. Eng. 2010, 36, 1007–1016. [Google Scholar] [CrossRef]
- Torrijos, V.; Gonzalo, O.G.; Trueba-Santiso, A.; Ruiz, I.; Soto, M. Effect of By-Pass and Effluent Recirculation on Nitrogen Removal in Hybrid Constructed Wetlands for Domestic and Industrial Wastewater Treatment. Water Res. 2016, 103, 92–100. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, G.; Ng, W.J.; Tan, S.K. A Review on Removing Pharmaceutical Contaminants from Wastewater by Constructed Wetlands: Design, Performance and Mechanism. Sci. Total Environ. 2014, 468–469, 908–932. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Zambello, E. How Efficient Are Constructed Wetlands in Removing Pharmaceuticals from Untreated and Treated Urban Wastewaters? A Review. Sci. Total Environ. 2014, 470–471, 1281–1306. [Google Scholar] [CrossRef]
- Ávila, C.; Matamoros, V.; Reyes-Contreras, C.; Piña, B.; Casado, M.; Mita, L.; Rivetti, C.; Barata, C.; García, J.; Bayona, J.M. Attenuation of Emerging Organic Contaminants in a Hybrid Constructed Wetland System under Different Hydraulic Loading Rates and Their Associated Toxicological Effects in Wastewater. Sci. Total Environ. 2014, 470–471, 1272–1280. [Google Scholar] [CrossRef]
- Ávila, C.; Nivala, J.; Olsson, L.; Kassa, K.; Headley, T.; Mueller, R.A.; Bayona, J.M.; García, J. Emerging Organic Contaminants in Vertical Subsurface Flow Constructed Wetlands: Influence of Media Size, Loading Frequency and Use of Active Aeration. Sci. Total Environ. 2014, 494–495, 211–217. [Google Scholar] [CrossRef]
- Reyes-Contreras, C.; Matamoros, V.; Ruiz, I.; Soto, M.; Bayona, J.M. Evaluation of PPCPs Removal in a Combined Anaerobic Digester-Constructed Wetland Pilot Plant Treating Urban Wastewater. Chemosphere 2011, 84, 1200–1207. [Google Scholar] [CrossRef]
- Álvarez, J.A.; Ruíz, I.; Soto, M. Anaerobic Digesters as a Pretreatment for Constructed Wetlands. Ecol. Eng. 2008, 33, 54–67. [Google Scholar] [CrossRef]
- Wu, S.; Kuschk, P.; Brix, H.; Vymazal, J.; Dong, R. Development of Constructed Wetlands Inperformance Intensifications for Wastewater Treatment: A Nitrogen and Organic Matter Targeted Review. Water Res. 2014, 57, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Fernández del Castillo, A.; Garibay, M.V.; Senés-Guerrero, C.; Orozco-Nunnelly, D.A.; de Anda, J.; Gradilla-Hernández, M.S. A Review of the Sustainability of Anaerobic Reactors Combined with Constructed Wetlands for Decentralized Wastewater Treatment. J. Clean. Prod. 2022, 133428. [Google Scholar] [CrossRef]
- Gonzalo, O.G.; Ruiz, I.; Soto, M. Integrating Pretreatment and Denitrification in Constructed Wetland Systems. Sci. Total Environ. 2017, 584–585, 1300–1309. [Google Scholar] [CrossRef]
- Moreira, F.C.; Soler, J.; Alpendurada, M.F.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Tertiary Treatment of a Municipal Wastewater toward Pharmaceuticals Removal by Chemical and Electrochemical Advanced Oxidation Processes. Water Res. 2016, 105, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Litter, M.; Quici, N. Photochemical Advanced Oxidation Processes for Water and Wastewater Treatment. Recent Patents Eng. 2011, 4, 217–241. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An Overview on the Advanced Oxidation Processes Applied for the Treatment of Water Pollutants Defined in the Recently Launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Glaze, W.H. Drinking-Water Treatment with Ozone. Environ. Sci. Technol. 1987, 21, 224–230. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical Review of Advanced Oxidation Processes in Organic Wastewater Treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Gonzalo, O.G.; Marín, Z.; Fernández, M.I.; Santaballa, J.A.; Ruiz, I.; Torres, E.; Canle, M.; Soto, M. Combination of Constructed Wetlands and Photodegradation Processes for the Elimination of Persistent Organic Pollutants from Municipal Wastewater, Proceedings of the 14th International IWA Conference on Sustainable Solutions for Small Water and Wastewater Treatment Systems (S2Small2017), Nantes, France, 22–26 October 2017; IWA Publishing: London, UK, 2017. [Google Scholar]
- Sánchez, M.; Fernández, M.I.; Ruiz, I.; Canle, M.; Soto, M. Persistent Organic Pollutants Removal during the Treatment of Municipal Wastewater in a System That Combines Anaerobic Digester, Constructed Wetland and Photodegradation Processes, Proceedings of the 16th International Conference of the IWA Specialist Group on Wetland Systems for Water Pollution Control, Valencia, Spain, 30 September–4 October 2018; IWA Publishing: London, UK, 2018. [Google Scholar]
- Sánchez, M.; Ramos, D.R.; Fernández, M.I.; Ruiz, I.; Canle, M.; Soto, M. Removal of Persistent Organic Pollutants by a 3-Step System: Anaerobic Digester, Vertical Flow Constructed Wetland and Different Post-Treatments. In Proceedings of the 9th International Symposium on Wetland Pollutant Dynamics and Control, Vienna, Austria, 13–17 September 2021. [Google Scholar]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar Photocatalysis: Materials, Reactors, Some Commercial, and Pre-Industrialized Applications. A Comprehensive Approach. Appl. Catal. B Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- IUPAC International Union of Pure and Applied Chemistry. Available online: https://iupac.org/ (accessed on 11 May 2021).
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium Dioxide in Our Everyday Life; Is It Safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Rodríguez, A.; Matamoros, V.; Fontàs, C.; Salvadó, V. The Ability of Biologically Based Wastewater Treatment Systems to Remove Emerging Organic Contaminants—A Review. Environ. Sci. Pollut. Res. 2014, 21, 11708–11728. [Google Scholar] [CrossRef] [PubMed]
- PubChem. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 31 May 2021).
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of Human Pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment Technologies for Emerging Contaminants in Wastewater Treatment Plants: A Review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on Fate and Mechanism of Removal of Pharmaceutical Pollutants from Wastewater Using Biological Approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Gin, K.Y.H. Occurrence and Removal of Pharmaceuticals, Hormones, Personal Care Products, and Endocrine Disrupters in a Full-Scale Water Reclamation Plant. Sci. Total Environ. 2017, 599–600, 1503–1516. [Google Scholar] [CrossRef]
- Faria, C.V.; Moreira, G.C.; Araújo, A.P.B.; Marques, L.E.; Oliveira, L.P.; Ricci, B.C.; Amaral, M.C.S.; Fonseca, F.V. Integration of Ozonation and an Anaerobic Expanded Granular Sludge Bed Reactor for Micropollutant Removal from Sewage. Environ. Sci. Pollut. Res. 2021, 28, 23778–23790. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Nazari, M.T.; De Souza, C.F.; Cadore, J.S.; Brião, V.B.; Piccin, J.S. Alternative Techniques for Caffeine Removal from Wastewater: An Overview of Opportunities and Challenges. J. Water Process Eng. 2020, 35, 101231. [Google Scholar] [CrossRef]
- Kümmerer, K. The Presence of Pharmaceuticals in the Environment Due to Human Use—Present Knowledge and Future Challenges. J. Environ. Manage. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Cunningham, V.L. Special Characteristics of Pharmaceuticals Related to Environmental Fate. In Pharmaceuticals in the Environment; Kümmerer, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 13–24. [Google Scholar]
- Liu, Z.-H.; Ogejo, J.A.; Pruden, A.; Knowlton, K.F. Occurrence, Fate and Removal of Synthetic Oral Contraceptives (SOCs) in the Natural Environment: A Review. Sci. Total Environ. 2011, 409, 5149–5161. [Google Scholar] [CrossRef]
- Joss, A.; Zabczynski, S.; Göbel, A.; Hoffmann, B.; Löffler, D.; McArdell, C.S.; Ternes, T.A.; Thomsen, A.; Siegrist, H. Biological Degradation of Pharmaceuticals in Municipal Wastewater Treatment: Proposing a Classification Scheme. Water Res. 2006, 40, 1686–1696. [Google Scholar] [CrossRef]
- Taoufik, N.; Boumya, W.; Janani, F.Z.; Elhalil, A.; Mahjoubi, F.Z.; Barka, N. Removal of Emerging Pharmaceutical Pollutants: A Systematic Mapping Study Review. J. Environ. Chem. Eng. 2020, 8, 104251. [Google Scholar] [CrossRef]
- Dhangar, K.; Kumar, M. Tricks and Tracks in Removal of Emerging Contaminants from the Wastewater through Hybrid Treatment Systems: A Review. Sci. Total Environ. 2020, 738, 140320. [Google Scholar] [CrossRef] [PubMed]
- Alvarino, T.; Torregrosa, N.; Omil, F.; Lema, J.M.; Suarez, S. Assessing the Feasibility of Two Hybrid MBR Systems Using PAC for Removing Macro and Micropollutants. J. Environ. Manage. 2017, 203, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.I.; Akanyeti, I.; Semião, A.J.C. Micropollutant Sorption to Membrane Polymers: A Review of Mechanisms for Estrogens. Adv. Colloid Interface Sci. 2011, 164, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yamashita, N.; Park, C.; Shimono, T.; Takeuchi, D.M.; Tanaka, H. Removal Characteristics of Pharmaceuticals and Personal Care Products: Comparison between Membrane Bioreactor and Various Biological Treatment Processes. Chemosphere 2017, 179, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, M.D.; Santos, J.L.; Aparicio, I.; Alonso, E. Distribution and Temporal Evolution of Pharmaceutically Active Compounds alongside Sewage Sludge Treatment. Risk Assessment of Sludge Application onto Soils. J. Environ. Manage. 2012, 102, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and Distribution of Pharmaceuticals in Wastewater and Sewage Sludge of the Conventional Activated Sludge (CAS) and Advanced Membrane Bioreactor (MBR) Treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef]
- Reyes-Contreras, C.; Neumann, P.; Barriga, F.; Venegas, M.; Domínguez, C.; Bayona, J.M.; Vidal, G. Organic Micropollutants in Sewage Sludge: Influence of Thermal and Ultrasound Hydrolysis Processes Prior to Anaerobic Stabilization. Environ. Technol. 2020, 41, 1358–1365. [Google Scholar] [CrossRef]
- Alvarino, T.; Suarez, S.; Lema, J.; Omil, F. Understanding the Sorption and Biotransformation of Organic Micropollutants in Innovative Biological Wastewater Treatment Technologies. Sci. Total Environ. 2018, 615, 297–306. [Google Scholar] [CrossRef]
- Sophia, A.C.; Lima, E.C. Removal of Emerging Contaminants from the Environment by Adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef]
- Alvarino, T.; Suarez, S.; Lema, J.M.; Omil, F. Understanding the Removal Mechanisms of PPCPs and the Influence of Main Technological Parameters in Anaerobic UASB and Aerobic CAS Reactors. J. Hazard. Mater. 2014, 278, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Achermann, S.; Helbling, D.E.; Johnson, D.R.; Fenner, K. Relative Contribution of Ammonia Oxidizing Bacteria and Other Members of Nitrifying Activated Sludge Communities to Micropollutant Biotransformation. Water Res. 2017, 109, 217–226. [Google Scholar] [CrossRef]
- Yi, T.; Harper, W.F.; Holbrook, R.D.; Love, N.G. Role of Particle Size and Ammonium Oxidation in Removal of 17α-Ethinyl Estradiol in Bioreactors. J. Environ. Eng. 2006, 132, 1527–1529. [Google Scholar] [CrossRef]
- Tran, N.H.; Urase, T.; Kusakabe, O. The Characteristics of Enriched Nitrifier Culture in the Degradation of Selected Pharmaceutically Active Compounds. J. Hazard. Mater. 2009, 171, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Pomiès, M.; Choubert, J.M.; Wisniewski, C.; Miège, C.; Budzinski, H.; Coquery, M. Lab-Scale Experimental Strategy for Determining Micropollutant Partition Coefficient and Biodegradation Constants in Activated Sludge. Environ. Sci. Pollut. Res. 2015, 22, 4383–4395. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fernandez, M.I.; de la Vega, P.T.M.; Jaramillo-Morán, M.A.; Garrido, M. Hybrid Constructed Wetland to Improve Organic Matter and Nutrient Removal. Water 2020, 12, 2023. [Google Scholar] [CrossRef]
- De la Varga, D.; Díaz, M.A.; Ruiz, I.; Soto, M. Avoiding Clogging in Constructed Wetlands by Using Anaerobic Digesters as Pre-Treatment. Ecol. Eng. 2013, 52, 262–269. [Google Scholar] [CrossRef]
- Stasinakis, A.S. Review on the Fate of Emerging Contaminants during Sludge Anaerobic Digestion. Bioresour. Technol. 2012, 121, 432–440. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Ternes, T.; Lema, J.M. Fate of Pharmaceutical and Personal Care Products (PPCPs) during Anaerobic Digestion of Sewage Sludge. Water Res. 2007, 41, 2139–2150. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Frouda, C.; Fountoulakis, M.S.; Drillia, P.; Kornaros, M.; Lyberatos, G. Pharmaceuticals and Health Care Products in Wastewater Effluents: The Example of Carbamazepine. Water Sci. Technol. Water Supply 2003, 3, 131–137. [Google Scholar] [CrossRef]
- Adrian, N.R.; Suflita, J.M. Anaerobic Biodegradation of Halogenated and Nonhalogenated N-, S-, and O-heterocyclic Compounds in Aquifer Slurries. Environ. Toxicol. Chem. 1994, 13, 1551–1557. [Google Scholar] [CrossRef]
- Lahti, M.; Oikari, A. Microbial Transformation of Pharmaceuticals Naproxen, Bisoprolol, and Diclofenac in Aerobic and Anaerobic Environments. Arch. Environ. Contam. Toxicol. 2011, 61, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Butkovskyi, A.; Sevenou, L.; Meulepas, R.J.W.; Leal, L.H.; Zeeman, G.; Rijnaarts, H.H.M.; Hernandez, L.; Wetsus, L. Micropollutant Removal from Black Water and Grey Water Sludge in a UASB-GAC Reactor. Water Sci. Technol. 2018, 77, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, F.B.; Brandt, E.M.F.; Aquino, S.F.; Chernicharo, C.A.L.; Afonso, R.J.C.F. Occurrence of Pharmaceuticals and Endocrine Disruptors in Raw Sewage and Their Behavior in UASB Reactors Operated at Different Hydraulic Retention Times. Water Sci. Technol. 2012, 66, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Kanjo, Y.; Mizutani, S. Removal Mechanisms for Endocrine Disrupting Compounds (EDCs) in Wastewater Treatment—Physical Means, Biodegradation, and Chemical Advanced Oxidation: A Review. Sci. Total Environ. 2009, 407, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.N.P.; Bui, X.T.; Nguyen, T.M.H.; Koottatep, T.; Bandyopadhyay, A. Insights of the Removal Mechanisms of Pharmaceutical and Personal Care Products in Constructed Wetlands. Curr. Pollut. Reports 2018, 4, 93–103. [Google Scholar] [CrossRef]
- Rabello, V.M.; Teixeira, L.C.R.S.; Gonçalves, A.P.V.; de Sá Salomão, A.L. The Efficiency of Constructed Wetlands and Algae Tanks for the Removal of Pharmaceuticals and Personal Care Products (PPCPs): A Systematic Review. Water. Air. Soil Pollut. 2019, 230, 1–12. [Google Scholar] [CrossRef]
- Ilyas, H.; Van Hullebusch, E.D. Role of Design and Operational Factors in the Removal of Pharmaceuticals by Constructed Wetlands. Water 2019, 11, 2356. [Google Scholar] [CrossRef]
- Cardinal, P.; Anderson, J.C.; Carlson, J.C.; Low, J.E.; Challis, J.K.; Beattie, S.A.; Bartel, C.N.; Elliott, A.D.; Montero, O.F.; Lokesh, S.; et al. Macrophytes May Not Contribute Significantly to Removal of Nutrients, Pharmaceuticals, and Antibiotic Resistance in Model Surface Constructed Wetlands. Sci. Total Environ. 2014, 482–483, 294–304. [Google Scholar] [CrossRef]
- Zhang, D.; Ni, W.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Performance Characterization of Pharmaceutical Removal by Horizontal Subsurface Flow Constructed Wetlands Using Multivariate Analysis. Clean—Soil Air Water 2015, 43, 1181–1189. [Google Scholar] [CrossRef]
- Hu, X.; Xie, H.; Zhuang, L.; Zhang, J.; Hu, Z.; Liang, S.; Feng, K. A Review on the Role of Plant in Pharmaceuticals and Personal Care Products (PPCPs) Removal in Constructed Wetlands. Sci. Total Environ. 2021, 780, 146637. [Google Scholar] [CrossRef] [PubMed]
- Mathon, B.; Ferreol, M.; Coquery, M.; Choubert, J.M.; Chovelon, J.M.; Miège, C. Direct Photodegradation of 36 Organic Micropollutants under Simulated Solar Radiation: Comparison with Free-Water Surface Constructed Wetland and Influence of Chemical Structure. J. Hazard. Mater. 2021, 407, 124801. [Google Scholar] [CrossRef] [PubMed]
- Mathon, B.; Coquery, M.; Miège, C.; Vandycke, A.; Choubert, J.M. Influence of Water Depth and Season on the Photodegradation of Micropollutants in a Free-Water Surface Constructed Wetland Receiving Treated Wastewater. Chemosphere 2019, 235, 260–270. [Google Scholar] [CrossRef]
- Chen, Y.; Vymazal, J.; Březinová, T.; Koželuh, M.; Kule, L.; Huang, J.; Chen, Z. Occurrence, Removal and Environmental Risk Assessment of Pharmaceuticals and Personal Care Products in Rural Wastewater Treatment Wetlands. Sci. Total Environ. 2016, 566–567, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Phong Vo, H.N.P.; Le, G.K.; Nguyen, T.M.H.; Bui, X.T.; Nguyen, K.H.; Rene, E.R.; Vo, T.D.H.; Cao, N.D.T.; Mohan, R. Acetaminophen Micropollutant: Historical and Current Occurrences, Toxicity, Removal Strategies and Transformation Pathways in Different Environments. Chemosphere 2019, 236, 124391. [Google Scholar] [CrossRef]
- Ávila, C.; Bayona, J.M.; Martín, I.; Salas, J.J.; García, J. Emerging Organic Contaminant Removal in a Full-Scale Hybrid Constructed Wetland System for Wastewater Treatment and Reuse. Ecol. Eng. 2015, 80, 108–116. [Google Scholar] [CrossRef]
- de Oliveira, M.; Atalla, A.A.; Frihling, B.E.F.; Cavalheri, P.S.; Migliolo, L.; Filho, F.J.C.M. Ibuprofen and Caffeine Removal in Vertical Flow and Free-Floating Macrophyte Constructed Wetlands with Heliconia Rostrata and Eichornia Crassipes. Chem. Eng. J. 2019, 373, 458–467. [Google Scholar] [CrossRef]
- Ilyas, H.; van Hullebusch, E.D. Performance Comparison of Different Types of Constructed Wetlands for the Removal of Pharmaceuticals and Their Transformation Products: A Review. Environ. Sci. Pollut. Res. 2020, 27, 14342–14364. [Google Scholar] [CrossRef]
- Vymazal, J.; Dvořáková Březinová, T.; Koželuh, M.; Kule, L. Occurrence and Removal of Pharmaceuticals in Four Full-Scale Constructed Wetlands in the Czech Republic—The First Year of Monitoring. Ecol. Eng. 2017, 98, 354–364. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Campos, L.C. Removal of Selected Emerging PPCP Compounds Using Greater Duckweed (Spirodela Polyrhiza) Based Lab-Scale Free Water Constructed Wetland. Water Res. 2017, 126, 252–261. [Google Scholar] [CrossRef] [Green Version]
- Ranieri, E.; Verlicchi, P.; Young, T.M. Paracetamol Removal in Subsurface Flow Constructed Wetlands. J. Hydrol. 2011, 404, 130–135. [Google Scholar] [CrossRef]
- Zhu, T.; Su, Z.; Lai, W.; Zhang, Y.; Liu, Y. Insights into the Fate and Removal of Antibiotics and Antibiotic Resistance Genes Using Biological Wastewater Treatment Technology. Sci. Total Environ. 2021, 776, 145906. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.S.; Su, H.C.; Ying, G.G.; Liu, F.; Liu, S.S.; He, L.Y.; Chen, Z.F.; Yang, Y.Q.; Chen, F.R. Removal of Antibiotics and Antibiotic Resistance Genes in Rural Wastewater by an Integrated Constructed Wetland. Environ. Sci. Pollut. Res. 2015, 22, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Feng, G.; Gao, X.; Sun, C.; Guo, J.S.; Zhu, Z. Removal of Pharmaceutically Active Compounds (PhACs) and Toxicological Response of Cyperus Alternifolius Exposed to PhACs in Microcosm Constructed Wetlands. J. Hazard. Mater. 2016, 301, 566–575. [Google Scholar] [CrossRef]
- Chen, J.; Wei, X.D.; Liu, Y.S.; Ying, G.G.; Liu, S.S.; He, L.Y.; Su, H.C.; Hu, L.X.; Chen, F.R.; Yang, Y.Q. Removal of Antibiotics and Antibiotic Resistance Genes from Domestic Sewage by Constructed Wetlands: Optimization of Wetland Substrates and Hydraulic Loading. Sci. Total Environ. 2016, 565, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Dordio, A.; Carvalho, A.J.P.; Teixeira, D.M.; Dias, C.B.; Pinto, A.P. Removal of Pharmaceuticals in Microcosm Constructed Wetlands Using Typha Spp. and LECA. Bioresour. Technol. 2010, 101, 886–892. [Google Scholar] [CrossRef]
- Ávila, C.; García-Galán, M.J.; Borrego, C.M.; Rodríguez-Mozaz, S.; García, J.; Barceló, D. New Insights on the Combined Removal of Antibiotics and ARGs in Urban Wastewater through the Use of Two Configurations of Vertical Subsurface Flow Constructed Wetlands. Sci. Total Environ. 2021, 755, 142554. [Google Scholar] [CrossRef]
- A, D.; Zhang, X.; Dai, Y.; Chen, C.; Yang, Y. Occurrence and Removal of Quinolone, Tetracycline, and Macrolide Antibiotics from Urban Wastewater in Constructed Wetlands. J. Clean. Prod. 2020, 252, 119677. [Google Scholar] [CrossRef]
- Miao, X.S.; Yang, J.J.; Metcalfe, C.D. Carbamazepine and Its Metabolites in Wastewater and in Biosolids in a Municipal Wastewater Treatment Plant. Environ. Sci. Technol. 2005, 39, 7469–7475. [Google Scholar] [CrossRef]
- Petrie, B.; Rood, S.; Smith, B.D.; Proctor, K.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Biotic Phase Micropollutant Distribution in Horizontal Sub-Surface Flow Constructed Wetlands. Sci. Total Environ. 2018, 630, 648–657. [Google Scholar] [CrossRef]
- Kahl, S.; Nivala, J.; van Afferden, M.; Müller, R.A.; Reemtsma, T. Effect of Design and Operational Conditions on the Performance of Subsurface Flow Treatment Wetlands: Emerging Organic Contaminants as Indicators. Water Res. 2017, 125, 490–500. [Google Scholar] [CrossRef]
- Nivala, J.; Kahl, S.; Boog, J.; van Afferden, M.; Reemtsma, T.; Müller, R.A. Dynamics of Emerging Organic Contaminant Removal in Conventional and Intensified Subsurface Flow Treatment Wetlands. Sci. Total Environ. 2019, 649, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, K.H.; Lee, E.; Lee, S.; Cho, J. Sorption of Pharmaceuticals to Soil Organic Matter in a Constructed Wetland by Electrostatic Interaction. Sci. Total Environ. 2018, 635, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sutton, N.B.; Lei, Y.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Fate and Distribution of Pharmaceutically Active Compounds in Mesocosm Constructed Wetlands. J. Hazard. Mater. 2018, 357, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Hijosa-Valsero, M.; Matamoros, V.; Sidrach-Cardona, R.; Martín-Villacorta, J.; Bécares, E.; Bayona, J.M. Comprehensive Assessment of the Design Configuration of Constructed Wetlands for the Removal of Pharmaceuticals and Personal Care Products from Urban Wastewaters. Water Res. 2010, 44, 3669–3678. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Bayona, J.M. Elimination of Pharmaceuticals and Personal Care Products in Subsurface Flow Constructed Wetlands. Environ. Sci. Technol. 2006, 40, 5811–5816. [Google Scholar] [CrossRef] [PubMed]
- Hijosa-Valsero, M.; Matamoros, V.; Martín-Villacorta, J.; Bécares, E.; Bayona, J.M. Assessment of Full-Scale Natural Systems for the Removal of PPCPs from Wastewater in Small Communities. Water Res. 2010, 44, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Hua, T.; Gersberg, R.M.; Zhu, J.; Ng, W.J.; Tan, S.K. Fate of Caffeine in Mesocosms Wetland Planted with Scirpus Validus. Chemosphere 2013, 90, 1568–1572. [Google Scholar] [CrossRef]
- Matamoros, V.; Duhec, A.; Albaigés, J.; Bayona, J.M. Photodegradation of Carbamazepine, Ibuprofen, Ketoprofen and 17α-Ethinylestradiol in Fresh and Seawater. Water. Air. Soil Pollut. 2009, 196, 161–168. [Google Scholar] [CrossRef]
- Lin, A.Y.C.; Reinhard, M. Photodegradation of Common Environmental Pharmaceuticals and Estrogens in River Water. Environ. Toxicol. Chem. 2005, 24, 1303–1309. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Jing, R.; Feng, X.; Dai, Y.; Tao, R.; Vymazal, J.; Cai, N.; Yang, Y. Removal of Acidic Pharmaceuticals by Small-Scale Constructed Wetlands Using Different Design Configurations. Sci. Total Environ. 2018, 639, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Gersberg, R.M.; Hua, T.; Zhu, J.; Tuan, N.A.; Tan, S.K. Pharmaceutical Removal in Tropical Subsurface Flow Constructed Wetlands at Varying Hydraulic Loading Rates. Chemosphere 2012, 87, 273–277. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Gersberg, R.M.; Zhu, J.; Hua, T.; Jinadasa, K.B.S.N.; Tan, S.K. Batch versus Continuous Feeding Strategies for Pharmaceutical Removal by Subsurface Flow Constructed Wetland. Environ. Pollut. 2012, 167, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Arias, C.; Brix, H.; Bayona, J.M. Preliminary Screening of Small-Scale Domestic Wastewater Treatment Systems for Removal of Pharmaceutical and Personal Care Products. Water Res. 2009, 43, 55–62. [Google Scholar] [CrossRef]
- Reyes-Contreras, C.; Hijosa-Valsero, M.; Sidrach-Cardona, R.; Bayona, J.M.; Bécares, E. Temporal Evolution in PPCP Removal from Urban Wastewater by Constructed Wetlands of Different Configuration: A Medium-Term Study. Chemosphere 2012, 88, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, H.; Gebhardt, W.; Linnemann, V.; Du Laing, G.; Rousseau, D.P.L. Laboratory- and Full-Scale Studies on the Removal of Pharmaceuticals in an Aerated Constructed Wetland: Effects of Aeration and Hydraulic Retention Time on the Removal Efficiency and Assessment of the Aquatic Risk. Water Sci. Technol. 2017, 76, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Hijosa-Valsero, M.; Sidrach-Cardona, R.; Martín-Villacorta, J.; Cruz Valsero-Blanco, M.; Bayona, J.M.; Bécares, E. Statistical Modelling of Organic Matter and Emerging Pollutants Removal in Constructed Wetlands. Bioresour. Technol. 2011, 102, 4981–4988. [Google Scholar] [CrossRef]
- He, Y.; Langenhoff, A.A.M.; Sutton, N.B.; Rijnaarts, H.H.M.; Blokland, M.H.; Chen, F.; Huber, C.; Schröder, P. Metabolism of Ibuprofen by Phragmites Australis: Uptake and Phytodegradation. Environ. Sci. Technol. 2017, 51, 4576–4584. [Google Scholar] [CrossRef]
- Zhang, D.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Removal of Pharmaceuticals and Personal Care Products in Aquatic Plant-Based Systems: A Review. Environ. Pollut. 2014, 184, 620–639. [Google Scholar] [CrossRef]
- Kimura, K.; Hara, H.; Watanabe, Y. Removal of Pharmaceutical Compounds by Submerged Membrane Bioreactors (MBRs). Desalination 2005, 178, 135–140. [Google Scholar] [CrossRef]
- Ávila, C.; Reyes, C.; Bayona, J.M.; García, J. Emerging Organic Contaminant Removal Depending on Primary Treatment and Operational Strategy in Horizontal Subsurface Flow Constructed Wetlands: Influence of Redox. Water Res. 2013, 47, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Ávila, C.; Pedescoll, A.; Matamoros, V.; Bayona, J.M.; García, J. Capacity of a Horizontal Subsurface Flow Constructed Wetland System for the Removal of Emerging Pollutants: An Injection Experiment. Chemosphere 2010, 81, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Hua, T.; Gersberg, R.M.; Zhu, J.; Ng, W.J.; Tan, S.K. Fate of Diclofenac in Wetland Mesocosms Planted with Scirpus Validus. Ecol. Eng. 2012, 49, 59–64. [Google Scholar] [CrossRef]
- Packer, J.L.; Werner, J.J.; Latch, D.E.; McNeill, K.; Arnold, W.A. Photochemical Fate of Pharmaceuticals in the Environment: Naproxen, Diclofenac, Clofibric Acid, and Ibuprofen. Aquat. Sci. 2003, 65, 342–351. [Google Scholar] [CrossRef]
- Dordio, A.V.; Estêvão Candeias, A.J.; Pinto, A.P.; Teixeira da Costa, C.; Palace Carvalho, A.J. Preliminary Media Screening for Application in the Removal of Clofibric Acid, Carbamazepine and Ibuprofen by SSF-Constructed Wetlands. Ecol. Eng. 2009, 35, 290–302. [Google Scholar] [CrossRef]
- Dai, Y.N.; Tao, R.; Tai, Y.P.; Tam, N.F.Y.; Dan, A.; Yang, Y. Application of a Full-Scale Newly Developed Stacked Constructed Wetland and an Assembled Bio-Filter for Reducing Phenolic Endocrine Disrupting Chemicals from Secondary Effluent. Ecol. Eng. 2017, 99, 496–503. [Google Scholar] [CrossRef]
- Toro-Vélez, A.F.; Madera-Parra, C.A.; Peña-Varón, M.R.; Lee, W.Y.; Bezares-Cruz, J.C.; Walker, W.S.; Cárdenas-Henao, H.; Quesada-Calderón, S.; García-Hernández, H.; Lens, P.N.L. BPA and NP Removal from Municipal Wastewater by Tropical Horizontal Subsurface Constructed Wetlands. Sci. Total Environ. 2016, 542, 93–101. [Google Scholar] [CrossRef]
- Christofilopoulos, S.; Kaliakatsos, A.; Triantafyllou, K.; Gounaki, I.; Venieri, D.; Kalogerakis, N. Evaluation of a Constructed Wetland for Wastewater Treatment: Addressing Emerging Organic Contaminants and Antibiotic Resistant Bacteria. N. Biotechnol. 2019, 52, 94–103. [Google Scholar] [CrossRef]
- Papaevangelou, V.A.; Gikas, G.D.; Tsihrintzis, V.A.; Antonopoulou, M.; Konstantinou, I.K. Removal of Endocrine Disrupting Chemicals in HSF and VF Pilot-Scale Constructed Wetlands. Chem. Eng. J. 2016, 294, 146–156. [Google Scholar] [CrossRef]
- Carranza-Diaz, O.; Schultze-Nobre, L.; Moeder, M.; Nivala, J.; Kuschk, P.; Koeser, H. Removal of Selected Organic Micropollutants in Planted and Unplanted Pilot-Scale Horizontal Flow Constructed Wetlands under Conditions of High Organic Load. Ecol. Eng. 2014, 71, 234–245. [Google Scholar] [CrossRef]
- Gabet-Giraud, V.; Miège, C.; Choubert, J.M.; Ruel, S.M.; Coquery, M. Occurrence and Removal of Estrogens and Beta Blockers by Various Processes in Wastewater Treatment Plants. Sci. Total Environ. 2010, 408, 4257–4269. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barceló, D.; Al Aukidy, M.; Zambello, E. Removal of Selected Pharmaceuticals from Domestic Wastewater in an Activated Sludge System Followed by a Horizontal Subsurface Flow Bed—Analysis of Their Respective Contributions. Sci. Total Environ. 2013, 454–455, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Oulton, R.L.; Kohn, T.; Cwiertny, D.M. Pharmaceuticals and Personal Care Products in Effluent Matrices: A Survey of Transformation and Removal during Wastewater Treatment and Implications for Wastewater Management. J. Environ. Monit. 2010, 12, 1956–1978. [Google Scholar] [CrossRef]
- Conkle, J.L.; White, J.R.; Metcalfe, C.D. Reduction of Pharmaceutically Active Compounds by a Lagoon Wetland Wastewater Treatment System in Southeast Louisiana. Chemosphere 2008, 73, 1741–1748. [Google Scholar] [CrossRef]
- Araña, J.; Garriga i Cabo, C.; Fernández Rodríguez, C.; Herrera Melián, J.A.; Ortega Méndez, J.A.; Doña Rodríguez, J.M.; Pérez Peña, J. Combining TiO2-Photocatalysis and Wetland Reactors for the Efficient Treatment of Pesticides. Chemosphere 2008, 71, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Mahne, D.; Štangar, U.L.; Trebše, P.; Bulc, T.G. TiO2—Based Photocatalytic Treatment of Raw and Constructed-Wetland Pretreated Textile Wastewater. Int. J. Photoenergy 2012, 2012, 725692. [Google Scholar] [CrossRef]
- Chen, K.C.; Wang, Y.H.; Lu, Y.C. Treatment of Polluted Water for Reclamation Using Photocatalysis and Constructed Wetlands. Catal. Today 2011, 175, 276–282. [Google Scholar] [CrossRef]
- Li, Z.; Gulyas, H.; Jahn, M.; Gajurel, D.R.; Otterpohl, R. Greywater Treatment by Constructed Wetlands in Combination with TiO2-Based Photocatalytic Oxidation for Suburban and Rural Areas without Sewer System. Water Sci. Technol. 2004, 48, 101–106. [Google Scholar] [CrossRef]
- Gulyas, H.; Choromanski, P.; Muelling, N.; Furmanska, M. Toward Chemical-Free Reclamation of Biologically Pretreated Greywater: Solar Photocatalytic Oxidation with Powdered Activated Carbon. J. Clean. Prod. 2009, 17, 1223–1227. [Google Scholar] [CrossRef]
- Horn, T.B.; Zerwes, F.V.; Kist, L.T.; Machado, Ê.L. Constructed Wetland and Photocatalytic Ozonation for University Sewage Treatment. Ecol. Eng. 2014, 63, 134–141. [Google Scholar] [CrossRef]
- Teodoro, A.; Boncz, M.Á.; Paulo, P.L.; Junior, A.M. Desinfecção de Água Cinza Por Fotocatálise Heterogênea. Eng. Sanit. Ambient. 2017, 22, 1017–1026. [Google Scholar] [CrossRef]
- Tung, T.X.; Xu, D.; Zhang, Y.; Zhou, Q.; Wang, X.; Pan, Y.; Disong, C.; Ting, Z.; Wu, Z. Effective Purification in Constructed Wetlands Strontium-Doped TiO2 Coated on Porous Filter Media. Polish J. Environ. Stud. 2019, 28, 4437–4446. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Liu, X.; Guo, F.; Su, X.; He, Q. Influence of Titanium Dioxide Nanoparticles on Functionalities of Constructed Wetlands for Wastewater Treatment. Chem. Eng. J. 2018, 352, 655–663. [Google Scholar] [CrossRef]
- Lazar, M.; Varghese, S.; Nair, S. Photocatalytic Water Treatment by Titanium Dioxide: Recent Updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Palacios-Villarreal, C.; Manzano, M.; Blanco, E.; Ramírez del Solar, M.; Levchuk, I. Photocatalytic Degradation of Pharmaceutically Active Compounds (PhACs) in Urban Wastewater Treatment Plants Effluents under Controlled and Natural Solar Irradiation Using Immobilized TiO2. Sol. Energy 2020, 208, 480–492. [Google Scholar] [CrossRef]
- He, Y.; Sutton, N.B.; Rijnaarts, H.H.H.; Langenhoff, A.A.M. Degradation of Pharmaceuticals in Wastewater Using Immobilized TiO2 Photocatalysis under Simulated Solar Irradiation. Appl. Catal. B Environ. 2016, 182, 132–141. [Google Scholar] [CrossRef]
- Cardoso-Vera, J.D.; Elizalde-Velázquez, G.A.; Islas-Flores, H.; Mejía-García, A.; Ortega-Olvera, J.M.; Gómez-Oliván, L.M. A Review of Antiepileptic Drugs: Part 1 Occurrence, Fate in Aquatic Environments and Removal during Different Treatment Technologies. Sci. Total Environ. 2021, 768, 145487. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Y.; Doherty, L.; Hu, Y.; Hao, X. A Review of Incorporation of Constructed Wetland with Other Treatment Processes. Chem. Eng. J. 2015, 279, 220–230. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Chao, H.R.; Chen, K.C. Treatment of Organic Matter and Tetracycline in Water by Using Constructed Wetlands and Photocatalysis. Appl. Sci. 2019, 9, 2680. [Google Scholar] [CrossRef]
- Felis, E.; Sochacki, A.; Magiera, S. Degradation of Benzotriazole and Benzothiazole in Treatment Wetlands and by Artificial Sunlight. Water Res. 2016, 104, 441–448. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A Critical Review of Advanced Oxidation Processes for Emerging Trace Organic Contaminant Degradation: Mechanisms, Factors, Degradation Products, and Effluent Toxicity. J. Water Process Eng. 2021, 40, 101778. [Google Scholar] [CrossRef]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of Water Matrix on the Removal of Micropollutants by Advanced Oxidation Technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef]
- Escolà Casas, M.; Matamoros, V. Novel Constructed Wetland Configurations for the Removal of Pharmaceuticals in Wastewater. Handb. Environ. Chem. 2020, 108, 163–190. [Google Scholar] [CrossRef]
- Melián, J.A.H.; Araña, J.; Ortega, J.A.; Muñoz, F.M.; Rendón, E.T.; Peña, J.P. Comparative Study of Phenolics Degradation between Biological and Photocatalytic Systems. J. Sol. Energy Eng. Trans. ASME 2008, 130, 0410031–0410037. [Google Scholar] [CrossRef]
- Berberidou, C.; Kitsiou, V.; Lambropoulou, D.A.; Antoniadis, A.; Ntonou, E.; Zalidis, G.C.; Poulios, I. Evaluation of an Alternative Method for Wastewater Treatment Containing Pesticides Using Solar Photocatalytic Oxidation and Constructed Wetlands. J. Environ. Manage. 2017, 195, 133–139. [Google Scholar] [CrossRef]
- Chow, K.L.; Man, Y.B.; Tam, N.F.Y.; Liang, Y.; Wong, M.H. Removal of Decabromodiphenyl Ether (BDE-209) Using a Combined System Involving TiO2 Photocatalysis and Wetland Plants. J. Hazard. Mater. 2017, 322, 263–269. [Google Scholar] [CrossRef]
- Koottatep, T.; Phong, V.H.N.; Chapagain, S.K.; Panuvatvanich, A.; Polprasert, C.; Ahn, K.H. Potential of Laterite Soil Coupling Fenton Reaction in Acetaminophen (ACT) Removal in Constructed Wetlands. Water. Air. Soil Pollut. 2017, 228, 1–9. [Google Scholar] [CrossRef]
- Vystavna, Y.; Frkova, Z.; Marchand, L.; Vergeles, Y.; Stolberg, F. Removal Efficiency of Pharmaceuticals in a Full Scale Constructed Wetland in East Ukraine. Ecol. Eng. 2017, 108, 50–58. [Google Scholar] [CrossRef]
- Conkle, J.L.; Lattao, C.; White, J.R.; Cook, R.L. Competitive Sorption and Desorption Behavior for Three Fluoroquinolone Antibiotics in a Wastewater Treatment Wetland Soil. Chemosphere 2010, 80, 1353–1359. [Google Scholar] [CrossRef]
- Yan, Q.; Xu, Y.; Yu, Y.; Zhu, Z.W.; Feng, G. Effects of Pharmaceuticals on Microbial Communities and Activity of Soil Enzymes in Mesocosm-Scale Constructed Wetlands. Chemosphere 2018, 212, 245–253. [Google Scholar] [CrossRef]
- Ilyas, H.; Masih, I.; van Hullebusch, E.D. The Anaerobic Biodegradation of Emerging Organic Contaminants by Horizontal Subsurface Flow Constructed Wetlands. Water Sci. Technol. 2021, 83, 2809–2828. [Google Scholar] [CrossRef] [PubMed]
- Hijosa-Valsero, M.; Matamoros, V.; Pedescoll, A.; Martín-Villacorta, J.; Bécares, E.; García, J.; Bayona, J.M. Evaluation of Primary Treatment and Loading Regimes in the Removal of Pharmaceuticals and Personal Care Products from Urban Wastewaters by Subsurface- Flow Constructed Wetlands. Int. J. Environ. Anal. Chem. 2011, 91, 632–653. [Google Scholar] [CrossRef]
- Matamoros, V.; Caselles-Osorio, A.; García, J.; Bayona, J.M. Behaviour of Pharmaceutical Products and Biodegradation Intermediates in Horizontal Subsurface Flow Constructed Wetland. A Microcosm Experiment. Sci. Total Environ. 2008, 394, 171–176. [Google Scholar] [CrossRef]
- Matamoros, V.; García, J.; Bayona, J.M. Behavior of Selected Pharmaceuticals in Subsurface Flow Constructed Wetlands: A Pilot-Scale Study. Environ. Sci. Technol. 2005, 39, 5449–5454. [Google Scholar] [CrossRef] [PubMed]
- Sharif, F.; Westerhoff, P.; Herckes, P. Impact of Hydraulic and Carbon Loading Rates of Constructed Wetlands on Contaminants of Emerging Concern (CECs) Removal. Environ. Pollut. 2014, 185, 107–115. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Reyes-Contreras, C.; Domínguez, C.; Bécares, E.; Bayona, J.M. Behaviour of Pharmaceuticals and Personal Care Products in Constructed Wetland Compartments: Influent, Effluent, Pore Water, Substrate and Plant Roots. Chemosphere 2016, 145, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Macci, C.; Peruzzi, E.; Doni, S.; Iannelli, R.; Masciandaro, G. Ornamental Plants for Micropollutant Removal in Wetland Systems. Environ. Sci. Pollut. Res. 2015, 22, 2406–2415. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Nguyen, L.X.; Arias, C.A.; Salvadó, V.; Brix, H. Evaluation of Aquatic Plants for Removing Polar Microcontaminants: A Microcosm Experiment. Chemosphere 2012, 88, 1257–1264. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, H. The Fate and Risk of Selected Pharmaceutical and Personal Care Products in Wastewater Treatment Plants and a Pilot-Scale Multistage Constructed Wetland System. Environ. Sci. Pollut. Res. 2014, 21, 1466–1479. [Google Scholar] [CrossRef]
- Francini, A.; Mariotti, L.; Di Gregorio, S.; Sebastiani, L.; Andreucci, A. Removal of Micro- Pollutants from Urban Wastewater by Constructed Wetlands with Phragmites Australis and Salix Matsudana. Environ. Sci. Pollut. Res. 2018, 25, 36474–36484. [Google Scholar] [CrossRef]
- Matamoros, V.; García, J.; Bayona, J.M. Organic Micropollutant Removal in a Full-Scale Surface Flow Constructed Wetland Fed with Secondary Effluent. Water Res. 2008, 42, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Salvadó, V. Evaluation of the Seasonal Performance of a Water Reclamation Pond-Constructed Wetland System for Removing Emerging Contaminants. Chemosphere 2012, 86, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Březinova, T.; Vymazal, J.; Koželuh, M.; Kule, L. Occurrence and Removal of Ibuprofen and Its Metabolites in Full-Scale Constructed Wetlands Treating Municipal Wastewater. Ecol. Eng. 2018, 120, 1–5. [Google Scholar] [CrossRef]
- Matamoros, V.; Arias, C.A.; Brix, H.; Bayona, J.M. Removal of Pharmaceuticals and Personal Care Products (PPCPs) from Urban Wastewater in a Pilot Vertical Flow Constructed Wetland and a Sand Filter. Environ. Sci. Technol. 2007, 41, 8171–8177. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhu, G.; Liu, Y.; Wu, B.; Ng, W.J.; Appan, A.; Tan, S.K. Phytoextraction, Phytotransformation and Rhizodegradation of Ibuprofen Associated with Typha Angustifolia in a Horizontal Subsurface Flow Constructed Wetland. Water Res. 2016, 102, 294–304. [Google Scholar] [CrossRef]
- Li, Y.; Wu, B.; Zhu, G.; Liu, Y.; Ng, W.J.; Appan, A.; Tan, S.K. High-Throughput Pyrosequencing Analysis of Bacteria Relevant to Cometabolic and Metabolic Degradation of Ibuprofen in Horizontal Subsurface Flow Constructed Wetlands. Sci. Total Environ. 2016, 562, 604–613. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, T.; Carvalho, P.N.; Zhang, L.; Arias, C.A.; Chen, Z.; Brix, H. Ibuprofen and Iohexol Removal in Saturated Constructed Wetland Mesocosms. Ecol. Eng. 2017, 98, 394–402. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Tan, S.K.; Gersberg, R.M.; Sadreddini, S.; Zhu, J.; Tuan, N.A. Removal of Pharmaceutical Compounds in Tropical Constructed Wetlands. Ecol. Eng. 2011, 37, 460–464. [Google Scholar] [CrossRef]
- Llorens, E.; Matamoros, V.; Domingo, V.; Bayona, J.M.; García, J. Water Quality Improvement in a Full-Scale Tertiary Constructed Wetland: Effects on Conventional and Specific Organic Contaminants. Sci. Total Environ. 2009, 407, 2517–2524. [Google Scholar] [CrossRef]
- Al-Rifai, J.H.; Khabbaz, H.; Schäfer, A.I. Removal of Pharmaceuticals and Endocrine Disrupting Compounds in a Water Recycling Process Using Reverse Osmosis Systems. Sep. Purif. Technol. 2011, 77, 60–67. [Google Scholar] [CrossRef]
- Campos, J.M.; Queiroz, S.C.N.; Roston, D.M. Removal of the Endocrine Disruptors Ethinyl Estradiol, Bisphenol A, and Levonorgestrel by Subsurface Constructed Wetlands. Sci. Total Environ. 2019, 693, 133514. [Google Scholar] [CrossRef] [PubMed]
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sánchez, R.; Ventura, F.; Petrovic, M.; Barcelo, D. Occurrence, Partition and Removal of Pharmaceuticals in Sewage Water and Sludge during Wastewater Treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar] [CrossRef] [PubMed]





| EOC | CWs | ADs | |||||
|---|---|---|---|---|---|---|---|
| Acronym | Total No. a | 2018–2021 Period | Included in This Review | Total No. a | 2018–2021 Period | Included in This Review | |
| Acetaminophen | ACE | 89 | 43 | 11 | 7 | 2 | 2 |
| Ofloxacin | OFL | 42 | 35 | 10 | 7 | 2 | 1 |
| Carbamazepine | CBZ | 203 | 98 | 18 | 22 | 16 | 6 |
| Caffeine | CAF | 110 | 51 | 19 | 14 | 7 | 1 |
| Ketoprofen | KET | 61 | 22 | 13 | 4 | 3 | 2 |
| Ibuprofen | IBU | 214 | 114 | 27 | 18 | 13 | 6 |
| Diclofenac | DCL | 174 | 89 | 22 | 35 | 22 | 7 |
| Clofibric acid | ACB | 110 | 26 | 7 | 5 | 2 | 1 |
| Bisphenol A | BPA | 137 | 80 | 12 | 32 | 19 | 1 |
| Sotalol | SOT | 5 | 2 | 5 | 1 | 1 | 1 |
| Total papers (without repeats) | 392 | 220 | 76 | 89 | 46 | 17 | |
| Name Acronym Chemical Composition | CAS MW b Formula | pKa a | log Kow b | log Dow c | kbio (L/gSS·d) d | kd (L/kgSS) d |
|---|---|---|---|---|---|---|
| Acetaminophen ACE  | 103-90-2 151.17 C8H9NO2 | 9.5 | 0.46 | 0.9 | 58–240 | 1.5–1160 |
| Ofloxacin OFL  | 82419-36-1 361.37 C18H20FN3O4 | 5.97 9.28 | −0.39 | - | 0.01–0.0933 | 12,000–22,100 |
| Caffeine CAF  | 58-08-2 194.19 C8H10N4O2 | 10.4 | −0.07 | −0.55 | 0.48–156.24 | <30–140 |
| Carbamazepine CBZ  | 298-46-4 236.27 C15H12N2O | 13.9 15.96 | 2.45 | 2.77 | 0.005–0.389 | <8–314 |
| Ketoprofen KET  | 22071-15-4 254.28 C16H14O3 | 4.45 | 3.12 | 0.39 | 0.24–3.36 | 0.24–226 |
| Ibuprofen IBU  | 15687-27-1 206.28 C13H18O2 | 4.85 | 3.5 3.97 | - | 3.24–38.7 | 6–103 |
| Diclofenac DCL  | 15307-86-5 296.15 C14H11Cl2NO2 | 4.2 | 4.98 | 2.26 0.86 | 0.02–8 | 1.9–321 |
| Clofibric Acid ACB  | 882-09-7 214.64 C10H11ClO3 | −4.9 3.2 | 2.57 | -0.42 | 0.03–1 | 7–87.5 |
| Bisphenol A BPA  | 80-05-7 228.29 C15H16O2 | 9.6 | 3.32 | 4.05 | 0.24–16.56 | 314–505 |
| Sotalol SOT  | 3930-20-9 272.37 C12H20N2O3S | 8.3 | 0.85 | - | - | - |
| All CW Types | Mean (n) for Each Type of CW | |||||
|---|---|---|---|---|---|---|
| Pollutant | Mean ± SD | n | CV (%) | SF | HF | VF |
| ACB | 43.0 ± 31.7 | 7 | 73.8 | 65.0 (2) | 48.8 (3) | 12.2 (2) |
| ACE | 89.9 ± 10.2 | 11 | 11.3 | 94.0 (2) | 86.7 (3) | 90.2 (5) |
| BPA | 53.3 ± 34.5 | 15 | 64.8 | 55.0 (2) | 46.9 (7) | 54.4 (5) |
| CAF | 89.0 ± 13.4 | 16 | 15.1 | 78.0 (2) | 91.3 (7) | 89.8 (6) |
| CBZ | 23.0 ± 22.0 | 13 | 95.6 | 40.5 (2) | 26.7 (6) | 7.0 (4) |
| DCL | 52.1 ± 22.4 | 23 | 43.0 | 56.8 (5) | 44.7 (9) | 52.9 (8) |
| IBU | 66.2 ± 29.7 | 23 | 44.8 | 49.7 (5) | 59.8 (9) | 80.8 (8) |
| KET | 62.3 ± 27.3 | 13 | 43.9 | 73.2 (3) | 58.9 (5) | 51.6 (4) |
| OFL | 81.3 ± 28.9 | 11 | 35.6 | 95.0 (1) | 89.4 (4) | 68.3 (5) |
| SOT | 19.4 ± 14.0 | 6 | 71.9 | 29.5 (2) | 13.8 (2) | 15.0 (2) |
| All pollutants | ||||||
| Minimum | 19.4 | 29.5 | 13.8 | 7.0 | ||
| Maximum | 89.9 | 95.0 | 91.3 | 90.2 | ||
| Mean | 58.0 | 63.7 | 56.7 | 52.2 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, M.; Ruiz, I.; Soto, M. The Potential of Constructed Wetland Systems and Photodegradation Processes for the Removal of Emerging Contaminants—A Review. Environments 2022, 9, 116. https://doi.org/10.3390/environments9090116
Sánchez M, Ruiz I, Soto M. The Potential of Constructed Wetland Systems and Photodegradation Processes for the Removal of Emerging Contaminants—A Review. Environments. 2022; 9(9):116. https://doi.org/10.3390/environments9090116
Chicago/Turabian StyleSánchez, Marta, Isabel Ruiz, and Manuel Soto. 2022. "The Potential of Constructed Wetland Systems and Photodegradation Processes for the Removal of Emerging Contaminants—A Review" Environments 9, no. 9: 116. https://doi.org/10.3390/environments9090116