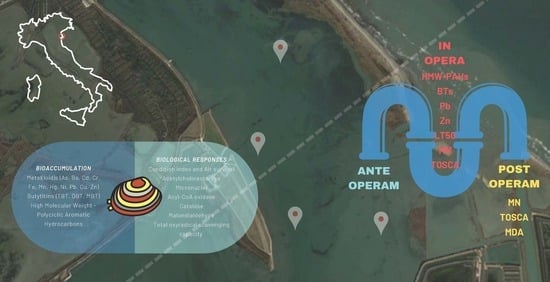
Before-During-After Biomonitoring Assessment for a Pipeline Construction in a Coastal Lagoon in the Northern Adriatic Sea (Italy)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Strategy
2.2. Analysis of Polycyclic Aromatics Hydrocarbons (PAHs)
2.3. Analysis of Heavy Metals and Metalloids
2.4. Analysis of Butyltins (BTs)
2.5. Biological Responses
2.6. Statistical Methods
3. Results
3.1. Contaminant Body Burdens
3.2. Biological Responses
3.3. Bioaccumulation and Biological Response Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morse, J.C.; Bae, Y.J.; Munkhjargal, G.; Sangpradub, N.; Tanida, K.; Vshivkova, T.S.; Wang, B.; Yang, L.; Yule, C.M. Freshwater biomonitoring with macroinvertebrates in East Asia. Front. Ecol. Environ. 2007, 5, 33–42. [Google Scholar] [CrossRef]
- Mezgebu, A. A review on freshwater biomonitoring with benthic invertebrates in Ethiopia. Environ. Sustain. Indic. 2022, 14, 100174. [Google Scholar] [CrossRef]
- Broeg, K.; Lehtonen, K.K. Indices for the assessment of environmental pollution of the Baltic Sea coasts: Integrated assessment of a multi-biomarker approach. Mar. Pollut. Bull. 2006, 53, 508–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milan, M.; Pauletto, M.; Patarnello, T.; Bargelloni, L.; Marin, M.G.; Matozzo, V. Gene transcription and biomarker responses in the clam Ruditapes philippinarum after exposure to ibuprofen. Aquat. Toxicol. 2013, 126, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Benedetti, M.; Giuliani, M.E.; Regoli, F. Oxidative metabolism of chemical pollutants in marine organisms: Molecular and biochemical biomarkers in environmental toxicology. Ann. N. Y. Acad. Sci. 2015, 1340, 8–19. [Google Scholar] [CrossRef]
- Rato, L.D.; Novais, S.C.; Lemos, M.F.L.; Alves, L.M.F.; Leandro, S.M. Homarus gammarus (Crustacea: Decapoda) larvae under an ocean acidification scenario: Responses across different levels of biological organization. Comp. Biochem. Physiol. 2017, 203, 29–38. [Google Scholar] [CrossRef]
- Lemos, M.F.L. Biomarker Studies in Stress Biology: From the Gene to Population, from the Organism to the Application. Biology 2021, 10, 1340. [Google Scholar] [CrossRef]
- Bryan, G.W.; Langston, W.J.; Hummerstone, L.G.; Burt, G.R. A guide to assessment of heavy metal contamination in estuaries using biological indicators. J. Mar. Biol. Assoc. UK 1985, 4, 1–92. [Google Scholar]
- Widdows, J.; Donkin, P. The application of combined tissue residue chemistry and physiological measurements of mussels (Mytilus edulis) for the assessment of environmental pollution. Hydrobiologia 1989, 188/189, 455–461. [Google Scholar] [CrossRef]
- Hopkin, S.P. In situ biological monitoring of pollution in terrestrial and aquatic ecosystem. In Handbook of Ecotoxicology; Blackwell Science Publication: London, UK, 1997; pp. 397–427. [Google Scholar] [CrossRef]
- Deplege, M.H.; Hopkin, S.P. Methods to assess effects on brackish, estuarine and near-coastal water organism. In Scope 53—Methods to Assess the Effects of Chemical on Ecosystems; John Wiley & Sons: Hoboken, NJ, USA, 1995; Volume 7, pp. 125–149. [Google Scholar]
- Dolbeth, M.; Crespo, D.; Leston, S.; Solan, M. Realistic scenarios of environmental disturbance lead to functionally importantchanges in benthic species-environment interactions. Mar. Environ. Res. 2019, 150, 104770. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.K. Use of biomarkers in environmental monitoring. Ocean Coast. Manag. 2009, 52, 348–354. [Google Scholar] [CrossRef]
- Gorbi, S.; Virno Lamberti, C.; Notti, A.; Benedetti, M.; Fattorini, D.; Moltedo, G.; Regoli, F. An ecotoxicological protocol with caged mussels, Mytilus galloprovincialis, for monitoring the impact of an offshore platform in the Adriatic Sea. Mar. Environ. Res. 2008, 65, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Bocchetti, R.; Regoli, F. Seasonal variability of oxidative biomarkers, lysosomal parameters, metallothioneins and peroxisomal enzymes in the Mediterranean mussel Mytilus galloprovincialis from Adriatic Sea. Chemosphere 2006, 65, 913–921. [Google Scholar] [CrossRef]
- Irato, P.; Santovito, G.; Cassini, A.; Piccinni, E.; Albergoni, V. Metal accumulation and binding protein induction in Mytilus galloprovincialis, Scapharca inaequivalvis, and Tapes philippinarum from the Lagoon of Venice. Arch. Environ. Contam. Toxicol. 2003, 44, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Boscolo Brusà, R.; Cacciatore, F.; Berto, D.; Marin, M.G.; Giani, M. Contamination of natural and cultured mussels (Mytilus galloprovincialis) from the northern Adriatic Sea by tributylin and dibutyltin compounds. Appl. Organomet. Chem. 2004, 18, 614–618. [Google Scholar] [CrossRef]
- Berto, D.; Boscolo Brusà, R.; Cacciatore, F.; Giani, M. Organotins used in antifouling paints: Environmental impact and contamination in a case study (Southern Venice Lagoon). Oceanol. Hydrobiol. Stud. 2006, 35, 270–283. [Google Scholar]
- Byrne, P.A.; O’Halloran, J. The impact of ballast effluent on the Manila clam Tapes semidecussatus. Ecotoxicology 2004, 13, 311–322. [Google Scholar] [CrossRef]
- Summer, K.H.; Halbach, S. I metalli. In Tossicologia; Zanichelli Editore: Bologna, Italy, 2000; Cap. 30; pp. 339–350. [Google Scholar]
- Samiullah, Y. Prediction of the Environmental Fate of Chemicals, 1st ed.; Springer: Dordrecht, The Netherlands, 1990; ISBN 978-1-85166-450-4. [Google Scholar] [CrossRef]
- Saulnier, I.; Mucci, A. Trace metal remobilization following the resuspension of estuarine sediments: Saguenay Fjord, Canada. J. Appl. Geochem. 2000, 15, 191–210. [Google Scholar] [CrossRef]
- Cantwell, M.G.; Burgess, R.M.; Kester, D.R. Release and phase partitioning of metals from anoxic estuarine sediments during periods of simulated resuspension. Environ. Sci. Technol. 2002, 36, 5328–5334. [Google Scholar] [CrossRef]
- Abel, P.D. Water Pollution Biology, 1st ed.; Ellis Horwood: Chichester, UK, 1989; ISBN 0745805124. [Google Scholar]
- Wijayaratne, R.D.; Means, J.C. Sorption of polycyclic aromatic hydrocarbons by natural estuarine colloids. Mar. Environ. Res. 1984, 11, 77–89. [Google Scholar] [CrossRef]
- Poulsen, R.; Gravert, T.K.O.; Tartara, A.; Bensen, H.K.; Gunnarsen, K.C.; Dicová, K.; Nielsen, N.J.; Christensen, J.H. A case study of PAH contamination using blue mussels as a bioindicator in a small Greenlandic fishing harbor. Mar. Pollut. Bull. 2021, 171, 112688. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, A.; Abondio, P.; Frapiccini, E.; Luiselli, D.; Marini, M. Meta-Analysis of a New Georeferenced Database on Polycyclic Aromatic Hydrocarbons in Western and Central Mediterranean Seafood. Appl. Sci. 2022, 12, 2776. [Google Scholar] [CrossRef]
- Stogiannidis, E.; Laane, R.; Whitacre David, M. Source Characterization of Polycyclic Aromatic Hydrocarbons by Using Their Molecular Indices: An Overview of Possibilities. In Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2015; Volume 234, Chapter 2; pp. 49–133. [Google Scholar] [CrossRef]
- Kennish, M.J. Practical Handbook of Estuarine and Marine Pollution, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997; ISBN 9780203742488. [Google Scholar] [CrossRef]
- Youngblood, W.W.; Blumer, M. Polycyclic aromatic hydrocarbons in the environment: Homologous series in soils and recent marine sediment. Geochim. Cosmochim. Acta 1975, 39, 1303–1314. [Google Scholar] [CrossRef]
- Alzieu, C. Impact of tributyltin on marine invertebrates. Ecotoxicology 2000, 9, 71–76. [Google Scholar] [CrossRef]
- Goldberg, E.D. TBT: An environmental dilemma. Environment 1986, 28, 17–44. [Google Scholar] [CrossRef]
- Noventa, S.; Berto, D.; Rampazzo, F.; Formalewicz, M.; Gion, C.; Boscolo Brusà, R.; Cacciatore, F.; Berducci, M.T.; Bianchi, J.F.; Onorati, F.; et al. TBT and antifouling strategies: The Italian and European legislation. Energia Ambiente e Innovazione ENEA, January–February 2014; pp. 89–94. [Google Scholar] [CrossRef]
- Blunden, S.J.; Bowen, H.J.M.; Hobbs, L.A.; Smith, P.J. The environmental chemistry of organotin compounds. In Environmental Chemistry; Bowen, H.J.M., Ed.; The Royal Society of Chemistry: London, UK, 1984; Volume 3, pp. 49–77. [Google Scholar] [CrossRef]
- Sousa, A.; Matsudaira, C.; Takahashi, S.; Tanabe, S.; Barroso, C. Integrative assessment of organotin contamination in a southern European estuarine system (Ria de Aveiro NWPortugal): Tracking temporal trends in order to evaluate the effectiveness of the EU ban. Mar. Pollut. Bull. 2007, 54, 1645–1653. [Google Scholar] [CrossRef]
- European Union. Water Framework Directive, Council Directive 2000/60/EC of 23 October 2000 Establishing a framework for Community action in the field of water policy. Off. J. Eur. Union 2000, 327, 1–72. [Google Scholar]
- Smolders, R.; Bervoets, L.; De Coen, W.; Blust, R. Cellular energy allocation in zebra mussels exposed along a pollution gradient: Linking cellular effects to higher levels of biological organization. Environ. Pollut. 2004, 129, 99–112. [Google Scholar] [CrossRef]
- Matozzo, V.; Marin, M.G. First evidence of altered vitellogenin-like protein levels in clam Tapes philippinarum and cockle Cerastoderma glaucum from the Lagoon of Venice. Mar. Pollut. Bull. 2007, 55, 494–504. [Google Scholar] [CrossRef]
- Nesto, N.; Romano, S.; Moschino, V.; Mauri, M.; Da Ros, L. Bioaccumulation and biomarker responses of trace metals and micro-organic pollutants in mussels and fish from the Lagoon of Venice, Italy. Mar. Pollut. Bull. 2007, 55, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Day, K.E.; Scott, I.M. Use of acetylcholinesterase activity to detect sublethal toxicity in stream invertebrates exposed to low concentrations of organophosphate insecticides. Aquat. Toxic. 1990, 18, 101–114. [Google Scholar] [CrossRef]
- Bocquené, G.; Galgani, F. Biological Effects of Contaminants: Cholinesterase Inhibition by Organophosphate and Carbamate Compounds. No. 22; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1998.
- Escartín, E.; Porte, C. The use of cholinesterase and carboxylesterase activities from Mytilus galloprovincialis in pollution monitoring. Environ. Toxicol. Chem. 1997, 16, 2090–2095. [Google Scholar] [CrossRef]
- Solé, M.; Baena, M.; Arnau, S.; Carrasson, M.; Maynou, F.; Cartes, J.E. Muscular cholinesterase activities and lipid peroxidation levels as biomarkers in several Mediterranean marine fish species and their relationship with ecological variables. Environ. Int. 2010, 36, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Gorbi, S.; Regoli, F. Total oxyradical scavenging capacity as an index of susceptibility to oxidative stress in marine organisms. Comments Toxicol. 2003, 9, 303–322. [Google Scholar] [CrossRef]
- Di Giulio, R.T.; Benson, W.H.; Sanders, B.M.; van Veld, P.A. Biochemical Mechanisms: Metabolism, Adaptation, and Toxicity. In Fundamentals of Aquatic Toxicology: Effects, Environmental Fate, and Risk Assessment, 2nd ed.; Rand, G.M., Ed.; Taylor and Francis: Abingdon, UK, 2003; pp. 523–560. [Google Scholar]
- European Union. Habitats Directive, Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, 206, 7–50. [Google Scholar]
- Simeoni, U.; Corbau, C. A review of the Delta Po evolution (Italy) related to climatic changes and human impacts. Geomorphology 2009, 107, 64–71. [Google Scholar] [CrossRef]
- Vincenzi, S.; De Leo, G.A.; Munari, C.; Mistri, M. Rapid estimation of potential yield for data-poor Tapes philippinarum fisheries in North Adriatic coastal lagoons. Hydrobiologia 2014, 724, 267–277. [Google Scholar] [CrossRef]
- Nasci, C.; Da Ros, L.; Nesto, N.; Sperni, L.; Passarini, F.; Pavoni, B. Biochemical and histochemical responses to environmental contaminants in clam, Tapes philippinarum, transplanted to different polluted areas of Venice Lagoon, Italy. Mar. Environ. Res. 2000, 50, 425–430. [Google Scholar] [CrossRef]
- Bortoli, A.; Troncon, A.; Dariol, S.; Pellizzato, F.; Pavoni, B. Butyltins and phenyltins in biota and sediments from the Lagoon of Venice. Oceanologia 2003, 45, 7–23. [Google Scholar]
- Marin, M.G.; Boscolo Brusà, R.; Cella, A.; Degetto, S.; Da Ros, L. Field validation of autometallographical black silver deposit (BSD) extent in three bivalve species from the Lagoon of Venice, Italy (Mytilus galloprovincialis, Tapes philippinarum, Scapharca inaequivalvis) for metal bioavailability assessment. Sci. Total Environ. 2006, 371, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Virno Lamberti, C.; Tomassetti, P.; Ceracchi, S.; Gabellini, M. Water Column Study in the Monitoring Plan of the First Italian Offshore LNG Terminal. Int. J. Environ. Sci. 2020, 26, 556187. [Google Scholar]
- Virno Lamberti, C.; Gabellini, M.; Maggi, C.; Nonnis, O.; Manfra, L.; Ceracchi, S.; Trabucco, B.; Moltedo, G.; Onorati, F.; Franceschini, G.; et al. An Envrionmental Monitoring Plan for the Construction and Operation of a Marine Terminal for Regasifying Liquefied Natural Gas (LNG) in North Adriatic Sea; Hughes, T.B., Ed.; Nova Publisher: New York, NY, USA, 2013; Volume 5, pp. 115–134. [Google Scholar]
- Cacciatore, F.; Bernarello, V.; Boscolo Brusà, R.; Sesta, G.; Franceschini, G.; Maggi, C.; Gabellini, M.; Virno Lamberti, C. PAH (Polycyclic Aromatic Hydrocarbon) bioaccumulation and PAHs/shell weight index in Ruditapes philippinarum (Adams & Reeve, 1850) from the Vallona lagoon (northern Adriatic Sea, NE Italy). Ecotoxicol. Environ. Safe 2018, 148, 787–798. [Google Scholar] [CrossRef]
- Maggi, C.; Berducci, M.T.; Di Lorenzo, B.; Dattolo, M.; Cozzolino, A.; Mariotti, S.; Fabrizi, V.; Spaziani, R.; Virno Lamberti, C. Temporal evolution of the environmental quality of the Vallona Lagoon (Northern Mediterranean, Adriatic Sea). Mar. Pollut. Bull. 2017, 125, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Binato, G.; Biancotto, G.; Piro, R.; Angeletti, R. Atomic absorption spectrometric screening and gas chromatographic-mass spectrometric determination of organotin compounds in marine mussels: An application in samples from the Venetian Lagoon. Fresenius. J. Anal. Chem. 1998, 361, 333–337. [Google Scholar] [CrossRef]
- Morabito, R.; Chiavarini, S.; Cremisini, C. Speciation of organotin compounds in environmental samples by GC-MS. In Quality Assurance for Environmental Analysis; Quevauviller, P., Maier, E.A., Griepink, B., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 17, pp. 435–464. [Google Scholar] [CrossRef]
- Morabito, R. Metodo per la determinazione di composti organostannici in sedimenti e matrici biologiche tramite GC -MS e GC-FPD. In Metodologie Analitiche di Riferimento del Programma di Monitoraggio per il Controllo Dell’ambiente Marino Costiero (Triennio 2001–2003); Cicero, A.M., Di Girolamo, I., Eds.; Ministero Dell’ambiente e Della Tutela del Territorio, ICRAM: Rome, Italy, 2001; Appendice I. [Google Scholar]
- Boscolo, R.; Cornello, M.; Giovanardi, O. Condition index and air survival time to compare three kind of Manila clam Tapes philippinarum (Adams & Reeve) farming systems. Aquac. Int. 2003, 11, 243–254. [Google Scholar] [CrossRef]
- Costa, S.; Coppola, F.; Pretti, C.; Intorre, L.; Meucci, V.; Soares, A.M.V.M.; Freitas, R.; Solé, M. The influence of climate change related factors on the response of two clam species to diclofenac. Ecotoxicol. Environ. Saf. 2020, 189, 109899. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Vienna, Austria. 2022. Available online: https://www.R-project.org/ (accessed on 13 May 2022).
- Bocchetti, R.; Virno Lamberti, C.; Pisanelli, B.; Razzetti, E.M.; Maggi, C.; Catalano, B.; Sesta, G.; Martuccio, G.; Gabellini, M.; Regoli, F. Seasonal variations of exposure biomarkers, oxidative stress responses and cell damage in the clams, Tapes philippinarum, and mussels, Mytilus galloprovincialis, from Adriatic sea. Mar. Environ. Res. 2008, 66, 24–26. [Google Scholar] [CrossRef]
- Eklund, B.; Eklund, D. Pleasure Boatyard Soils are Often Highly Contaminated. Environ. Manag. 2014, 53, 930–946. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.K.; Takahashi, S.; Min, B.Y.; Tanabe, S. Butyltin residues in blue mussels (Mytilus edulis) and ark shells (Scapharca broughtonii) collected from Korean coastal waters. Environ. Pollut. 2002, 117, 475–486. [Google Scholar] [CrossRef]
- Corsolini, S.; Mazzoni, M.; Polesello, S.; Rusconi, M.; Valsecchi, S. Perfluorinated alkyl acids in bivalves, water, and sediments of the Po river Delata (Adriatic Sea). Organohalogen Compd. 2014, 76, 684–687. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission to assess the health risks to consumers associated with exposure to organotins in foodstuffs (Question N EFSA-Q-2003-110). Adopted on 22 September 2004. EFSA J. 2004, 102, 1–119. Available online: http://www.efsa.eu.int.1/119 (accessed on 23 June 2022).
- Belfroid, A.C.; Purperhart, M.; Ariese, F. Organotin Levels in Seafood. Mar. Pollut. Bull. 2000, 40, 226–232. [Google Scholar] [CrossRef]
- Airaksinen, R.; Rantakokko, P.; Turunen, A.W.; Vartiainen, T.; Vuorinen, P.J.; Lappalainen, A.; Vihervuori, A.; Mannio, J.; Hallikainen, A. Organotin intake through fish consumption in Finland. Environ. Res. 2010, 110, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Moon, H.B.; Choi, H.G. Intake and potential health risk of Butyltin compounds from seafood consumption in Korea. Arch. Environ. Contam. Toxicol. 2012, 62, 333–340. [Google Scholar] [CrossRef]
- Marin, M.G.; Moschino, V.; Deppieri, M.; Lucchetta, L. Variations in gross biochemical composition, energy value and condition index of T. philippinarum from the Lagoon of Venice. Aquaculture 2003, 219, 859–871. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Tchounwou, P.B. Serum Acetyl Cholinesterase as a Biomarker of Arsenic Induced Neurotoxicity in Sprague-Dawley Rats. Int. J. Environ. Res. Public Health 2005, 2, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Schiavo, S.; Xiangli, D.; Rametta, G.; Miglietta, M.L.; Oliviero, M.; Changwen, W.; Manzo, S. Early ecotoxic effects of ZnO nanoparticle chronic exposure in Mytilus galloprovincialis revealed by transcription of apoptosis and antioxidant-related genes. Ecotoxicology 2018, 27, 369–384. [Google Scholar] [CrossRef]




| PCA Groups of Samples | Bioaccumulation | Biological Responses | |
|---|---|---|---|
| AO vs. IO vs. PO | AO and IO partially overlapped, PO differed | AO, IO, and PO overlapped | |
| Ante operam | November 2005 | Mn, Fe, Cr, Ni and Ba (L022V, L023V) (↑) | |
| February 2006 | HMW-PAH, TBT, DBT (↑) | - | |
| In opera | February 2007 | HMW-PAH, TBT, DBT, Cd, Hg (↑) | CI (↑) |
| June 2008 | - | CI, (↑) | |
| Post operam | November 2011 | Cu, Zn (↑) | |
| June 2012 | - | MDA (↑) | |
| November 2012 | LT50 (↑) | ||
| May 2013 | Cd, Hg, Pb (↓) | AOX, AChE (↑) | |
| November 2013 | Cu, Zn, As (↑) | TOSC_ROO·, TOSC_HO· (↑) | |
| June 2014 | Fe, Mn, Cu, Zn (↓) | CI (↑) LT50(↓) | |
| October 2014 | HMW-PAH (↓) Cu, Zn (↑) | TOSC_ROO·, TOSC_HO· (↑) | |
| June 2015 | HMW-PAH (↓) (↑) | TOSC_HO·, CAT, AOX (↑) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacciatore, F.; Moltedo, G.; Bernarello, V.; Formalewicz, M.; Catalano, B.; Martuccio, G.; Benedetti, M.; Berducci, M.T.; Sesta, G.; Franceschini, G.; et al. Before-During-After Biomonitoring Assessment for a Pipeline Construction in a Coastal Lagoon in the Northern Adriatic Sea (Italy). Environments 2022, 9, 81. https://doi.org/10.3390/environments9070081
Cacciatore F, Moltedo G, Bernarello V, Formalewicz M, Catalano B, Martuccio G, Benedetti M, Berducci MT, Sesta G, Franceschini G, et al. Before-During-After Biomonitoring Assessment for a Pipeline Construction in a Coastal Lagoon in the Northern Adriatic Sea (Italy). Environments. 2022; 9(7):81. https://doi.org/10.3390/environments9070081
Chicago/Turabian StyleCacciatore, Federica, Ginevra Moltedo, Valentina Bernarello, Malgorzata Formalewicz, Barbara Catalano, Giacomo Martuccio, Maura Benedetti, Maria Teresa Berducci, Giulio Sesta, Gianluca Franceschini, and et al. 2022. "Before-During-After Biomonitoring Assessment for a Pipeline Construction in a Coastal Lagoon in the Northern Adriatic Sea (Italy)" Environments 9, no. 7: 81. https://doi.org/10.3390/environments9070081