Uncovering the Hidden Dangers of Microplastic Pollution in Lake Ecosystems: Effects of Ingestion on Talitrid Amphipods
Abstract
:1. Introduction
2. Methods
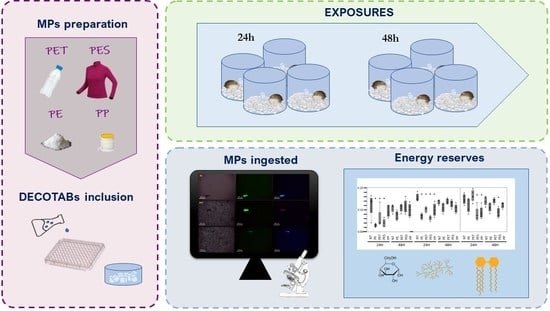
2.1. Preparation of MPs
2.2. Food Preparation
2.3. Exposure Conditions
2.4. Statistical Analysis
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2022: An Analysis of European Plastics Production, Demand and End-of-Life Management; Plastic Europe: Paris, France, 2022; pp. 1–81. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 7 February 2023).
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.A.; Robison, B.H.; Gagne, T.O.; Erwin, B.; Firl, E.; Halden, R.U.; Hamilton, J.A.; Katija, K.; Lisin, S.E.; Rolsky, C.; et al. The vertical distribution and biological transport of marine microplastics across the epipelagic and mesopelagic water column. Sci. Rep. 2019, 9, 7843. [Google Scholar] [CrossRef] [Green Version]
- Courtene-Jones, W.; Quinn, B.; Gary, S.F.; Mogg, A.O.M.; Narayanaswamy, B.E. Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the Rockall Trough, North Atlantic Ocean. Environ. Pollut. 2017, 231, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Lombardini, E.; Martellini, T.; Katsoyiannis, A.; Fossi, M.C.; Corsolini, S. Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR. Chemosphere 2017, 175, 391–400. [Google Scholar] [CrossRef]
- Iannilli, V.; Pasquali, V.; Setini, A.; Corami, F. First evidence of microplastics ingestion in benthic amphipods from Svalbard. Environ. Res. 2019, 179, 108811. [Google Scholar] [CrossRef] [PubMed]
- Iannilli, V.; Passatore, L.; Carloni, S.; Lecce, F.; Sciacca, G.; Zacchini, M.; Pietrini, F. Microplastic Toxicity and Trophic Transfer in Freshwater Organisms: Ecotoxicological and Genotoxic Assessment in Spirodela polyrhiza (L.) Schleid. and Echinogammarus veneris (Heller, 1865) Treated with Polyethylene Microparticles. Water 2023, 15, 921. [Google Scholar] [CrossRef]
- McLachlan, A.; Defeo, O. The Ecology of Sandy Shores; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Griffiths, C.L.; Stenton-Dozey, J.M.E.; Koop, K. Kelp wrack and the flow of energy through a sandy beach ecosystem. In Sandy Beaches as Ecosystems; McLachlan, A., Erasmus, T., Eds.; W. Junk: The Hague, The Netherlands, 1983; pp. 547–556. [Google Scholar]
- Lastra, M.; de La Huz, R.; Sánchez-Mata, A.G.; Rodil, I.F.; Aerts, K.; Beloso, S.; López, J. Ecology of exposed sandy beaches in northern Spain: Environmental factors controlling macrofauna communities. J. Sea Res. 2006, 55, 128–140. [Google Scholar] [CrossRef]
- Ugolini, A.; Borghini, F.; Calosi, P.; Bazzicalupo, M.; Chelazzi, G.; Focardi, S. Mediterranean Talitrus saltator (Crustacea, Amphipoda) as a biomonitor of heavy metals contamination. Mar. Pollut. Bull. 2004, 48, 526–532. [Google Scholar] [CrossRef]
- Olabarria, C.; Incera, M.; Garrido, J.; Rodil, I.F.; Rossi, F. Intraspecific diet shift in Talitrus saltator inhabiting exposed sandy beaches. Estuar. Coast. Shelf Sci. 2009, 84, 282–288. [Google Scholar] [CrossRef]
- Fanini, L.; Coleman, C.O.; Lowry, J.K. Insights into the ecology of Cryptorchestia garbinii on the shores of the urban lake Tegel (Berlin, Germany). Vie Milieu Life Environ. 2019, 69, 187–191. [Google Scholar]
- De Matthaeis, E.; Davolos, D.; Cobolli, M.; Ketmaier, V. Isolation by Distance in Equilibrium and Nonequilibrium Populations of Four Talitrid Species in the Mediterranean Sea. Evolution 2000, 54, 1606–1613. Available online: http://www.jstor.org/stable/2640659 (accessed on 1 April 2023).
- Ruffo, S.; Tarocco, M.; Latella, L. Cryptorchestia garbinii n. sp. (Amphipoda: Talitridae) from Lake Garda (Northern Italy), previously referred to as Orchestia cavimana Heller, 1865, and notes on the distribution of the two species. Ital. J. Zool. 2014, 81, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Davolos, D.; Vonk, R.; Latella, L.; De Matthaeis, E. The name of a model species: The case of Orchestia cavimana (Crustacea: Amhipoda: Talitridae). Eur. Zool. J. 2018, 85, 229–231. [Google Scholar] [CrossRef] [Green Version]
- Iannilli, V.; Corami, F.; Grasso, P.; Lecce, F.; Buttinelli, M.; Setini, A. Plastic abundance and seasonal variation on the shorelines of three volcanic lakes in Central Italy: Can amphipods help detect contamination? Environ. Sci. Pollut. Res. 2020, 27, 14711–14722. [Google Scholar] [CrossRef]
- Battistin, G.; Latella, L.; Iannilli, V. Microplastic pollution in the food web: Observation of ingestion by the talitrid amphipod Cryptorchestia garbinii on the shores of Lake Garda Microplastic pollution in the food web: Observation of ingestion by the talitrid amphipod Cryptorchestia garbinii. Eur. Zool. J. 2023, 90, 73–82. [Google Scholar] [CrossRef]
- Malygina, N.; Mitrofanova, E.; Kuryatnikova, N.; Biryukov, R.; Zolotov, D.; Pershin, D.; Chernykh, D. Microplastic pollution in the surface waters from plain and mountainous Lakes in Siberia, Russia. Water 2021, 13, 2287. [Google Scholar] [CrossRef]
- Hammer, J.; Kraak, M.H.S.; Parsons, J.R. Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2012; pp. 1–44. [Google Scholar]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.; Yu, H. Research status and prospects of microplastic pollution in lakes. Environ. Monit. Assess. 2023, 195, 485. [Google Scholar] [CrossRef]
- Park, J.H.; Hong Seungwoo Kim, O.H.; Kim, C.H.; Kim, J.; Kim, J.W.; Hong Sungguan Lee, H.J. Polypropylene microplastics promote metastatic features in human breast cancer. Sci. Rep. 2023, 13, 6252. [Google Scholar] [CrossRef]
- Kampfraath, A.A.; Hunting, E.R.; Mulder, C.; Breure, A.M.; Gessner, M.O.; Kraak, M.H.S.; Admiraal, W. DECOTAB: A multipurpose standard substrate to assess effects of litter quality on microbial decomposition and invertebrate consumption. Freshw. Sci. 2012, 31, 1156–1162. [Google Scholar] [CrossRef] [Green Version]
- Götz, A.; Imhof, H.K.; Geist, J.; Beggel, S. Moving Toward Standardized Toxicity Testing Procedures with Particulates by Dietary Exposure of Gammarids. Environ. Toxicol. Chem. 2021, 40, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Abdelrhman, K.F.A.; Bacci, G.; Nistri, A.; Mengoni, A.; Ugolini, A. Diet and gut microbiota of two supralittoral amphipods Orchestia montagui and Talitrus saltator living in different microhabitats. Estuar. Coast. Shelf Sci. 2017, 197, 119–125. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Straub, S.; Hirsch, P.E.; Burkhardt-Holm, P. Biodegradable and petroleum-based microplastics do not differ in their ingestion and excretion but in their biological effects in a freshwater invertebrate Gammarus fossarum. Int. J. Environ. Res. Public Health 2017, 14, 774. [Google Scholar] [CrossRef] [Green Version]
- van Handel, E. Rapid determination of glycogen and sugars in mosquitoes. J. Ofthe Am. Mosq. Control. Assoc. 1985, 1, 299–301. [Google Scholar]
- van Handel, E. Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control. Assoc. 1985, 1, 302–304. [Google Scholar]
- Foray, V.; Pelisson, P.F.; Bel-Venner, M.C.; Desouhant, E.; Venner, S.; Menu, F.; Giron, D.; Rey, B. A handbook for uncovering the complete energetic budget in insects: The van Handel’s method (1985) revisited. Physiol. Entomol. 2012, 37, 295–302. [Google Scholar] [CrossRef]
- Stanton, T.; Johnson, M.; Nathanail, P.; Gomes, R.L.; Needham, T.; Burson, A. Exploring the Efficacy of Nile Red in Microplastic Quantification: A Costaining Approach. Environ. Sci. Technol. Lett. 2019, 6, 606–611. [Google Scholar] [CrossRef]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef]
- O'Connor, J.D.; Mahon, A.M.; Ramsperger, A.F.; Trotter, B.; Redondo-Hasselerharm, P.E.; Koelmans, A.A.; Lally, H.T.; Murphy, S. Microplastics in Freshwater Biota: A Critical Review of Isolation, Characterization, and Assessment Methods. Glob. Chall. 2019, 4, 1800118. [Google Scholar] [CrossRef] [Green Version]
- Ríos, J.M.; Tesitore, G.; de Mello, F.T. Does color play a predominant role in the intake of microplastics fragments by freshwater fish: An experimental approach with Psalidodon eigenmanniorum. Environ. Sci. Pollut. Res. 2022, 29, 49457–49464. [Google Scholar] [CrossRef]
- DeMott, W.R. The role of taste in food selection by freshwater zooplankton. Oecologia 1986, 69, 334–340. [Google Scholar] [CrossRef] [PubMed]
- DeMott, W.R. Discrimination between algae and artificial particles by freshwater and marine copepods. Limnol. Oceanogr. 1988, 33, 397–408. [Google Scholar] [CrossRef]
- Scherer, C.; Weber, A.; Lambert, S.; Wagner, M. Interactions of microplastics with freshwater biota. In Freshwater Microplastics, 58th ed.; Wagner, M., Lambert, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 153–180. [Google Scholar] [CrossRef] [Green Version]
- Sanders, R.W.; Porter, K.G.; Bennett, S.J.; De Biase, A.E. Seasonal patterns of bacterivory by flagellates, ciliates, rotifers, and cladocerans in a freshwater planktonic community. Limnol. Oceanogr. 1989, 34, 673–687. [Google Scholar] [CrossRef] [Green Version]
- Thouvenot, A.; Richardot, M.; Debroas, D.; Devaux, J. Bacterivory of metazooplankton, ciliates and flagellates in a newly flooded reservoir. J. Plankton Res. 1999, 21, 1659–1679. [Google Scholar] [CrossRef] [Green Version]
- Blarer, P.; Burkhardt-Holm, P. Microplastics affect assimilation efficiency in the freshwater amphipod Gammarus fossarum. Environ. Sci. Pollut. Res. 2016, 23, 23522–23532. [Google Scholar] [CrossRef]
- Kratina, P.; Watts, T.J.; Green, D.S.; Kordas, R.L.; O’Gorman, E.J. Interactive effects of warming and microplastics on metabolism but not feeding rates of a key freshwater detritivore. Environ. Pollut. 2019, 255, 113259. [Google Scholar] [CrossRef]
- Shen, H.; Nzabanita, D.; Sinclair, G.M.; Vu, H.; Grist, S.; Nugegoda, D.; Long, S.M. Changes in metabolic profiles of amphipods Allorchestes compressa after acute exposures to copper, pyrene, and their mixtures. Environ. Toxicol. Pharmacol. 2023, 99, 104120. [Google Scholar] [CrossRef]
- Weber, A.; Scherer, C.; Brennholt, N.; Reifferscheid, G.; Wagner, M. PET microplastics do not negatively affect the survival, development, metabolism and feeding activity of the freshwater invertebrate Gammarus pulex. Environ. Pollut. 2018, 234, 181–189. [Google Scholar] [CrossRef]
- Rani-Borges, B.; Meitern, R.; Teesalu, P.; Raudna-Kristoffersen, M.; Kreitsberg, R.; Heinlaan, M.; Tuvikene, A.; Ivask, A. Effects of environmentally relevant concentrations of microplastics on amphipods. Chemosphere 2022, 309, 136599. [Google Scholar] [CrossRef]
- Moyo, S. An enigma: A meta-analysis reveals the effect of ubiquitous microplastics on different taxa in aquatic systems. Front. Environ. Sci. 2022, 10, 1–17. [Google Scholar] [CrossRef]
- Jiang, W.; Fang, J.; Du, M.; Gao, Y.; Fang, J.; Jiang, Z. Microplastics influence physiological processes, growth and reproduction in the Manila clam, Ruditapes philippinarum. Environ. Pollut. 2022, 293, 118502. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.; Aureli, F.; Iannilli, V. Bisphenols A and Its Analogues Induce Genotoxic Damage in Marine and Freshwater Amphipods. Environ. Adv. 2022, 7, 100183. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.N.; Liu, J.H.; Feng, X.S. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ. Pollut. 2018, 243, 462–471. [Google Scholar] [CrossRef]




| PE | PET * | PES | PP | |
|---|---|---|---|---|
| 24 h | 4.5 ± 0.7 | 3.3 ± 0.7 | 8 ± 2.4 | 1.8 ± 0.4 |
| 48 h | 7.1 ± 2.2 | 8.6 ± 1.6 | 7 ± 0.8 | 2.4 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciotti, C.; Setini, A.; Lecce, F.; Iannilli, V. Uncovering the Hidden Dangers of Microplastic Pollution in Lake Ecosystems: Effects of Ingestion on Talitrid Amphipods. Environments 2023, 10, 115. https://doi.org/10.3390/environments10070115
Ciotti C, Setini A, Lecce F, Iannilli V. Uncovering the Hidden Dangers of Microplastic Pollution in Lake Ecosystems: Effects of Ingestion on Talitrid Amphipods. Environments. 2023; 10(7):115. https://doi.org/10.3390/environments10070115
Chicago/Turabian StyleCiotti, Camilla, Andrea Setini, Francesca Lecce, and Valentina Iannilli. 2023. "Uncovering the Hidden Dangers of Microplastic Pollution in Lake Ecosystems: Effects of Ingestion on Talitrid Amphipods" Environments 10, no. 7: 115. https://doi.org/10.3390/environments10070115