Climate Change and Dispersal Ability Jointly Affects the Future Distribution of Crocodile Lizards
Abstract
:Simple Summary
Abstract
1. Introduction
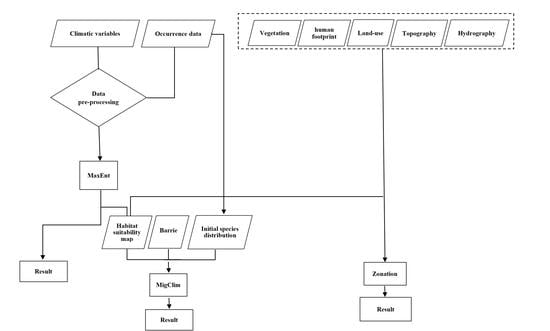
2. Methods
2.1. Occurrence Data
2.2. Climatic Variables
2.3. Modelling Procedures
2.4. Dispersal Analysis
2.5. Conservation Units
3. Results
3.1. Model Verification and Key Climatic Factors
3.2. LGM and Current Climate Scenario Analysis
3.3. Projected Future Change in Species Distributions
3.4. Dispersal Scenario Analysis
3.5. Conservation Units
4. Discussion
4.1. Key Factors Affecting S. Crocodilurus Distributions
4.2. Predicted Habitat Suitability
4.3. Conservation Units
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, J.B.; Shobrak, M.; Wilms, T.M.; Arif, I.A.; Khan, H.A. Climate change and animals in saudi arabia. Saudi J. Biol. Sci. 2012, 19, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E.M. Biodiversity: The ravages of guns, nets and bulldozers. Nature 2016, 536, 143–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPBES. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- Jetz, W.; Fine, P.V. Global gradients in vertebrate diversity predicted by historical area-productivity dynamics and contemporary environment. PLoS Biol. 2012, 10, e1001292. [Google Scholar] [CrossRef] [PubMed]
- Diele-Viegas, L.M.; Rocha, C.F.D. Unraveling the influences of climate change in lepidosauria (reptilia). J. Therm. Biol. 2018, 78, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Diele-Viegas, L.M.; Figueroa, R.T.; Vilela, B.; Rocha, C.F.D. Are reptiles toast? A worldwide evaluation of lepidosauria vulnerability to climate change. Clim. Chang. 2020, 159, 581–599. [Google Scholar] [CrossRef]
- Ramirez-Villegas, J.; Cuesta, F.; Devenish, C.; Peralvo, M.; Jarvis, A.; Arnillas, C.A. Using species distributions models for designing conservation strategies of tropical andean biodiversity under climate change. J. Nat. Conserv. 2014, 22, 391–404. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate change 2021: The physical science basis. In Future Global Climate: Scenario-42 Based Projections and Near-Term Information; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Zhang, L.X.; Chen, X.L.; Xin, X.G. Short commentary on cmip6 scenario model intercomparison project (scenariomip). Clim. Chang. Res. 2019, 15, 519–525. [Google Scholar]
- Stevens-Rumann, C.S.; Kemp, K.B.; Higuera, P.E.; Harvey, B.J.; Rother, M.T.; Donato, D.C.; Morgan, P.; Veblen, T.T. Evidence for declining forest resilience to wildfires under climate change. Ecol. Lett. 2018, 21, 243–252. [Google Scholar] [CrossRef]
- Franklin, J.; Serra-Diaz, J.M.; Syphard, A.D.; Regan, H.M. Global change and terrestrial plant community dynamics. Proc. Natl. Acad. Sci. USA 2016, 113, 3725–3734. [Google Scholar] [CrossRef] [Green Version]
- Abatzoglou, J.T.; Williams, A.P. Impact of anthropogenic climate change on wildfire across western us forests. Proc. Natl. Acad. Sci. USA 2016, 113, 11770–11775. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Edmands, S. Sex ratios in a warming world: Thermal effects on sex-biased survival, sex determination, and sex reversal. J. Hered. 2021, 112, 155–164. [Google Scholar] [CrossRef]
- Godwin, J.L.; Lumley, A.J.; Michalczyk, L.; Martin, O.Y.; Gage, M.J.G. Mating patterns influence vulnerability to the extinction vortex. Glob. Chang. Biol. 2020, 26, 4226–4239. [Google Scholar] [CrossRef]
- Jensen, M.P.; Allen, C.D.; Eguchi, T.; Bell, I.P.; LaCasella, E.L.; Hilton, W.A.; Hof, C.A.M.; Dutton, P.H. Environmental warming and feminization of one of the largest sea turtle populations in the world. Curr. Biol. 2018, 28, 154–159.e4. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, N.; Literman, R.; Neuwald, J.L.; Mizoguchi, B.; Iverson, J.B.; Riley, J.L.; Litzgus, J.D. Extreme thermal fluctuations from climate change unexpectedly accelerate demographic collapse of vertebrates with temperature-dependent sex determination. Sci. Rep. 2019, 9, 4254. [Google Scholar] [CrossRef] [Green Version]
- Loyola, R.D.; Lemes, P.; Faleiro, F.V.; Trindade-Filho, J.; Machado, R.B. Severe loss of suitable climatic conditions for marsupial species in brazil: Challenges and opportunities for conservation. PLoS ONE 2012, 7, e46257. [Google Scholar]
- Nori, J.; Carrasco, P.A.; Leynaud, G.C. Venomous snakes and climate change: Ophidism as a dynamic problem. Clim. Chang. 2013, 122, 67–80. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; Siqueira, M.F.d.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Lenoir, J.; Gegout, J.C.; Marquet, P.A.; Ruffray, P.d.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef]
- Sweet, L.C.; Green, T.; Heintz, J.G.; Frakes, N.; Graver, N.; Rangitsch, J.S.; Rodgers, J.E.; Heacox, S.; Barrows, C.W. Congruence between future distribution models and empirical data for an iconic species at joshua tree national park. Ecosphere 2019, 10, e02763. [Google Scholar] [CrossRef] [Green Version]
- Barrows, C.W.; Ramirez, A.; Sweet, L.C.; Morelli, T.L.; Millar, C.; Frakes, N.; Rodgers, J.; Mahalovich, M.F. Validating climate change refugia: Empirical bottom-up approaches to support management actions. Front. Ecol. Environ. 2020, 18, 298–306. [Google Scholar] [CrossRef]
- Wu, J. Can changes in the distribution of lizard species over the past 50 years be attributed to climate change? Theor. Appl. Climatol. 2016, 125, 785–798. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Rodriguez, N.; Kuchling, G.; Arnall, S.G.; Kearney, M.R. Reptile embryos and climate change: Modelling limits of viability to inform translocation decisions. Biol. Conserv. 2016, 204, 134–147. [Google Scholar] [CrossRef]
- Sinervo, B.; Méndez-de-la-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Cruz, M.V.-S.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of lizard diversity by climate change and altered thermal niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruge, A.; Alvarez, P.; Fontán, A.; Cotano, U.; Chust, G. Thermal niche tracking and future distribution of atlantic mackerel spawning in response to ocean warming. Front. Mar. Sci. 2016, 3, 86. [Google Scholar] [CrossRef] [Green Version]
- Burrows, M.T.; Schoeman, D.S.; Buckley, L.B.; Moore, P.; Poloczanska, E.S.; Brander, K.M.; Brown, C.; Bruno, J.F.; Duarte, C.M.; Halpern, B.S.; et al. The pace of shifting climate in marine and terrestrial ecosystems. Science 2011, 334, 652–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erauskin-Extramiana, M.; Arrizabalaga, H.; Hobday, A.J.; Cabre, A.; Ibaibarriaga, L.; Arregui, I.; Murua, H.; Chust, G. Large-scale distribution of tuna species in a warming ocean. Glob. Chang. Biol. 2019, 25, 2043–2060. [Google Scholar] [CrossRef] [Green Version]
- Monahan, W.B.; Tingley, M.W. Niche tracking and rapid establishment of distributional equilibrium in the house sparrow show potential responsiveness of species to climate change. PLoS ONE 2012, 7, e42097. [Google Scholar] [CrossRef] [Green Version]
- Pecl, G.; Araujo, M.; Bell, J.; Blanchard, J.; Bonebrake, T.; Chen, I.; Clark, T.; Colwell, R.; Danielsen, F.; Evengard, B. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Corkery, I.; Bell, B.D.; Nelson, N.J. Thermoregulation of a temperate reptile in a forested habitat. Zoology 2018, 127, 63–69. [Google Scholar] [CrossRef]
- Stanton-Jones, W.K.; Parusnath, S.; Alexander, G.J. The impact of posture and basking orientation on thermoregulation in the sungazer (smaug giganteus). J. Therm. Biol. 2018, 75, 45–53. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N. Habitat Suitability and Distribution Models; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Feng, X.; Park, D.S.; Walker, C.; Peterson, A.T.; Merow, C.; Papes, M. A checklist for maximizing reproducibility of ecological niche models. Nat. Ecol. Evol. 2019, 3, 1382–1395. [Google Scholar] [CrossRef]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Morán-Ordóñez, A.; Lahoz-Monfort, J.J.; Elith, J.; Wintle, B.A. Evaluating 318 continental-scale species distribution models over a 60-year prediction horizon: What factors influence the reliability of predictions? Glob. Ecol. Biogeogr. 2017, 26, 371–384. [Google Scholar] [CrossRef]
- Murray, J.V.; Low Choy, S.; McAlpine, C.A.; Possingham, H.P.; Goldizen, A.W. Evaluating model transferability for a threatened species to adjacent areas: Implications for rock-wallaby conservation. Austral Ecol. 2011, 36, 76–89. [Google Scholar] [CrossRef]
- Tessarolo, G.; Lobo, J.M.; Rangel, T.F.; Hortal, J. High uncertainty in the effects of data characteristics on the performance of species distribution models. Ecol. Indic. 2021, 121, 107147. [Google Scholar] [CrossRef]
- Araú, M.B.; Anderson, R.P.; Má, A.; Barbosa, R.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; et al. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar]
- Duputié, A.; Zimmermann, N.E.; Chuine, I. Where are the wild things? Why we need better data on species distribution. Glob. Ecol. Biogeogr. 2013, 23, 457–467. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is my species distribution model fit for purpose? Matching data and models to applications. Glob. Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Subba, B.; Sen, S.; Ravikanth, G.; Nobis, M.P. Direct modelling of limited migration improves projected distributions of himalayan amphibians under climate change. Biol. Conserv. 2018, 227, 352–360. [Google Scholar] [CrossRef]
- Nobis, M.P.; Normand, S. Kissmig—A simple model for r to account for limited migration in analyses of species distributions. Ecography 2014, 37, 1282–1287. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, L.; Nobis, M.P.; Wu, X.; Pan, K.; Wang, K.; Dakhil, M.A.; Du, M.; Xiong, Q.; Pandey, B.; et al. Climate change jointly with migration ability affect future range shifts of dominant fir species in southwest china. Divers. Distrib. 2019, 26, 352–367. [Google Scholar] [CrossRef]
- Xie, H.; Chen, L.; Huang, H.Y.; He, N.; Liu, H.Y.; Wu, Z.J. Research on movement pattern and the influencing factors of Shinisaurus crocodilurus in the Luokeng nature reserve, Guangdong, China. J. Guangxi Norm. Univ. 2017, 35, 106–113. [Google Scholar]
- Van Schingen, M.; Schepp, U.; Pham, C.T.; Nguyen, T.Q.; Ziegler, T. Last chance to see? A review of the threats to and use of the crocodile lizard. Traffic Bull 2015, 27, 19–26. [Google Scholar]
- Huang, C.M.; Yu, H.; Wu, Z.J.; Li, Y.B.; Wei, F.W.; Gong, M.H. Population and conservation strategies for the chinese crocodile lizard (shinisaurus crocodilurus) in china. Anim. Biodivers. Conserv. 2008, 31, 63–70. [Google Scholar]
- IUCN. The Iucn Red List of Threatened Species Version 2020-2; IUCN: Gran, Switzerland, 2020; Downloaded on 9 July 2020. [Google Scholar]
- He, N.; Zhang, X.L.; Que, Q.Q.; Chen, Z.N.; Wu, Z.J. Physiological thermoregulation of the chinese crocodile lizard shinisaurus crocodilurus in the luokeng nature reserve, guangdong. Chin. J. Wildl. 2021, 43, 139–144. [Google Scholar]
- Liang, W.B.; Zhang, Y.X.; Su, P.; Long, Q.; Huang, J.Q. Observation on time budget of Shinisaurus crocodilurus in captivity. Sichuan J. Zool. 2006, 25, 264–266. [Google Scholar]
- Ziegler, T.; Van Schingen, M.; Rauhaus, A.; Dang, P.H.; Pham, D.T.K.; Pham, C.T.; Nguyen, T.Q. New insights into the habitat use and husbandry of crocodile lizards (reptilia: Shinisauridae) including the conception of new facilities for vietnamese crocodile lizards shinisaurus crocodilurus vietnamensis in vietnam and germany. Int. Zoo Yearb. 2019, 53, 250–269. [Google Scholar] [CrossRef]
- Yu, H.; Huang, C.M.; Wu, Z.J.; Ning, J.J.; Dai, D.L. Observation on habit of chinese crocodilian lizard. Sichuan J. Zool. 2006, 2, 364–366. [Google Scholar]
- Zollweg, M.; Kühne, H. Krokodilschwanzechsen-Shinisaurus Crocodilurus; Natur und Tier: Münster, Germany, 2013. [Google Scholar]
- Cordero, G.A.; Telemeco, R.S.; Gangloff, E.J. Reptile embryos are not capable of behavioral thermoregulation in the egg. Evol. Dev. 2018, 20, 40–47. [Google Scholar] [CrossRef]
- Hall, J.M.; Sun, B. Heat tolerance of reptile embryos: Current knowledge, methodological considerations, and future directions. J. Exp. Zool. Parta. 2021, 335, 45–58. [Google Scholar] [CrossRef]
- Iknayan, K.J.; Beissinger, S.R. Collapse of a desert bird community over the past century driven by climate change. Proc. Natl. Acad. Sci. USA 2018, 115, 8597–8602. [Google Scholar] [CrossRef] [Green Version]
- Strona, G.; Bradshaw, C.J.A. Co-extinctions annihilate planetary life during extreme environmental change. Sci. Rep. 2018, 8, 16724. [Google Scholar] [CrossRef] [Green Version]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the coupled model intercomparison project phase 6 (cmip6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef] [Green Version]
- Cos, J.; Doblas-Reyes, F.; Jury, M.; Marcos, R.; Bretonnière, P.-A.; Samsó, M. The mediterranean climate change hotspot in the cmip5 and cmip6 projections. Earth Syst. Dyn. 2022, 13, 321–340. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y. Unveiling the conservation biogeography of a data-deficient endangered bird species under climate change. PLoS ONE 2014, 9, e84529. [Google Scholar] [CrossRef]
- Blach-Overgaard, A.; Svenning, J.-C.; Dransfield, J.; Greve, M.; Balslev, H. Determinants of palm species distributions across africa: The relative roles of climate, non-climatic environmental factors, and spatial constraints. Ecography 2010, 33, 380–391. [Google Scholar] [CrossRef]
- Wu, Z.J.; Dai, D.L.; Huang, C.M.; Yu, H.; Ning, J.J.; Zhong, Y.M. Selection of shinisaurus crocodilurus on forest type in mountain streams in luokeng nature reserve of guangdong province. China J. Ecol. 2007, 26, 1777–1781. [Google Scholar]
- Wu, Z.J.; Dai, D.L.; Nin, J.J.; Huang, C.M.; Yu, H. Seasonal differences in habitat selection of the crocodile lizard (Shinisaurus crocodilurus) in Luokeng nature reserve, Guangdong. Acta Ecol. Sin. 2012, 32, 4691–4699. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M.; Schapire, R. Maxent software for Modeling Species Niches and Distributions (Version 3.4.1). 2017. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 10 October 2021).
- Wang, D.; Cui, B.; Duan, S.; Chen, J.; Fan, H.; Lu, B.; Zheng, J. Moving north in china: The habitat of pedicularis kansuensis in the context of climate change. Sci. Total Environ. 2019, 697, 133979. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Thuiller, W.; Lavorel, S.; Araújo, M.B. Niche properties and geographical extent as predictors of species sensitivity to climate change. Glob. Ecol. Biogeogr. 2005, 14, 347–357. [Google Scholar] [CrossRef]
- Çoban, H.O.; Örücü, Ö.K.; Arslan, E.S. Maxent modeling for predicting the current and future potential geographical distribution of quercus libani olivier. Sustainability 2020, 12, 2671. [Google Scholar] [CrossRef] [Green Version]
- Swets, J. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- Engler, R.; Guisan, A. Migclim: Predicting plant distribution and dispersal in a changing climate. Divers. Distrib. 2009, 15, 590–601. [Google Scholar] [CrossRef]
- Engler, R.; Hordijk, W.; Guisan, A. The migclim r package—Seamless integration of dispersal constraints into projections of species distribution models. Ecography 2012, 35, 872–878. [Google Scholar] [CrossRef]
- Sen, S.; Shivaprakash, K.N.; Aravind, N.A.; Ravikanth, G.; Dayanandan, S. Ecological niche modeling for conservation planning of an endemic snail in the verge of becoming a pest in cardamom plantations in the western ghats biodiversity hotspot. Ecol. Evol. 2016, 6, 6510–6523. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Wang, C.; Han, S.; Yu, J. Planning the priority protected areas of endangered orchid species in northeastern China. Biodivers. Conserv. 2014, 23, 1395–1409. [Google Scholar] [CrossRef]
- Moilanen, A.; Arponen, A.; Leppänen, J.; Meller, L.; Kujala, H. Zonation: Spatial Conservation Planning Framework and Software Version 3.0 User Manual; University of Helsinki Press: Helsinki, Finland, 2011. [Google Scholar]
- Lehtomäki, J.; Moilanen, A. Methods and workflow for spatial conservation prioritization using zonation. Environ. Model. Softw. 2013, 47, 128–137. [Google Scholar] [CrossRef]
- Beltran, I.; Perry, C.; Degottex, F.; Whiting, M.J. Behavioral thermoregulation by mothers protects offspring from global warming but at a cost. Physiol Biochem. Zool. 2021, 94, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Black, I.R.G.; Aedy, L.K.; Tattersall, G.J. Hot and covered: How dragons face the heat and thermoregulate. J. Comp. Physiol. B 2021, 191, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Benton, M.J. Predicting biotic responses to future climate warming with classic ecogeographic rules. Curr. Biol. 2020, 30, R744–R749. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, G.J.; Arnaout, B.; Symonds, M.R.E. The evolution of the avian bill as a thermoregulatory organ. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1630–1656. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Krol, E. Maximal heat dissipation capacity and hyperthermia risk: Neglected key factors in the ecology of endotherms. J. Anim. Ecol. 2010, 79, 726–746. [Google Scholar] [CrossRef]
- McKechnie, A.E. Physiological and morphological effects of climate change. In Effects of Climate Change on Birds, 2nd ed.; Dunn, P.O., Møller, A.P., Eds.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Wooden, K.M.; Walsberg, G.E. Effect of environmental temperature on body temperature and metabolic heat production in a heterothermic rodent, spermophilus tereticaudus. J. Exp. Biol. 2002, 205, 2099–2105. [Google Scholar] [CrossRef]
- McKechnie, A.E.; Wolf, B.O. Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 2010, 6, 253–256. [Google Scholar] [CrossRef] [Green Version]
- Tingley, R.; Hitchmough, R.A.; Chapple, D.G. Life-history traits and extrinsic threats determine extinction risk in new zealand lizards. Biol. Conserv. 2013, 165, 62–68. [Google Scholar] [CrossRef]
- Xu, H.N.; Xiao, T.T.; Yang, M.X.; Zhang, W.Y. Analysis on the change characteristics of summer precipitation in southwest china. Adv. Geosci. 2019, 9, 908–920. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.S.Y.; Gillies, R.R.; Buckley, B.M.; Yoon, J.H.; Cho, C. Regional trends in early-monsoon rainfall over vietnam and ccsm4 attribution. Clim. Dyn. 2018, 52, 363–372. [Google Scholar] [CrossRef]
- Zhong, Y.X.; Chen, C.W.; Wang, Y.P. Biological and extrinsic correlates of extinction risk in chinese lizards. Curr. Zool. 2022, 68, 285–293. [Google Scholar] [CrossRef]
- Van Schingen, M.; Pham, C.; Thi, H.; Nguyen, T.; Bernardes, M.; Bonkowski, M.; Ziegler, T. First ecological assessment on the endangered crocodile lizard shinisaurus crocodilurus ahl, 1930 in vietnam: Microhabitat characterization and habitat selection. Herpetol. Conserv. Biol. 2015, 10, 948–958. [Google Scholar]
- Easterling, D.R.; Meehl, C.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef] [Green Version]
- Lele, S.; Springate-Baginski, O.; Lakerveld, R.; Deb, D.; Dash, P. Ecosystem services: Origins, contributions, pitfalls, and alternatives. Conserv. Soc. 2013, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Xu, X.; Yu, B.; Xu, C.; Liu, M.; Wang, K. Quantifying the impacts of climate and human activities on water and sediment discharge in a karst region of southwest china. J. Hydrol. 2016, 542, 836–849. [Google Scholar] [CrossRef]
- Zhou, Q.; Luo, Y.; Zhou, X.; Cai, M.; Zhao, C. Response of vegetation to water balance conditions at different time scales across the karst area of southwestern china-a remote sensing approach. Sci. Total Environ. 2018, 645, 460–470. [Google Scholar] [CrossRef]
- Nie, Y.P.; Chen, H.S.; Wang, K.L.; Yang, J. Water source utilization by woody plants growing on dolomite outcrops and nearby soils during dry seasons in karst region of southwest china. J. Hydrol. 2012, 420–421, 264–274. [Google Scholar] [CrossRef]
- Efe, R. Ecological properties of vegetation formations on karst terrains in the central taurus mountains (southern turkey). Procedia Soc. Behav. Sci. 2014, 120, 673–679. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, S.; Yamaura, T.; Kajikawa, Y. Influence of sub-mesoscale topography on daytime precipitation associated with thermally driven local circulations over a mountainous region. J. Atmos. Sci. 2021, 78, 2511–2532. [Google Scholar] [CrossRef]
- Liu, M.; Xu, X.; Wang, D.; Sun, A.Y.; Wang, K. Karst catchments exhibited higher degradation stress from climate change than the non-karst catchments in southwest china: An ecohydrological perspective. J. Hydrol. 2016, 535, 173–180. [Google Scholar] [CrossRef]
- Liu, M.X.; Xu, X.L. Ecohydrology in karst region of southwestern china under changing climate and human activities: A review. Res. Agric. Mod. 2018, 39, 930–936. [Google Scholar]
- Nori, J.; Moreno Azócar, D.L.; Cruz, F.B.; Bonino, M.F.; Leynaud, G.C. Translating niche features: Modelling differential exposure of argentine reptiles to global climate change. Austral Ecol. 2016, 41, 367–375. [Google Scholar] [CrossRef]
- Huang, H.Y.; Wang, H.; LI, L.M.; Wu, Z.J.; Chen, J.P. Genetic diversity and population demography of the chinese crocodile lizard (Shinisaurus crocodilurus) in China. PLoS ONE 2014, 9, e91570. [Google Scholar] [CrossRef] [Green Version]
- Van Schingen, M.; Duc Le, M.; Thi Ngo, H.; The Pham, C.; Quy Ha, Q.; Quang Nguyen, T.; Ziegler, T. Is there more than one crocodile lizard? An integrative taxonomic approach reveals vietnamese and chinese Shinisaurus crocodilurus represent separate conservation and taxonomic units. Der Zool. Gart. 2016, 85, 240–260. [Google Scholar] [CrossRef]
- Werner, C. Trophic Niche Selection of Freshwater Adapted Lizards and Salamanders in North Vietnam. Master’s Thesis, University of Cologne, Cologne, Germany, 2015. [Google Scholar]
- Zollweg, M. Erfolgreiches projekt zum schutz der krokodilschwanz-höckerechse in china. ZGAP Mitt. 2012, 28, 15. [Google Scholar]
- Sarremejane, R.; Mykrä, H.; Huttunen, K.L.; Mustonen, K.R.; Marttila, H.; Paavola, R.; Sippel, K.; Veijalainen, N.; Muotka, T. Climate-driven hydrological variability determines inter-annual changes in stream invertebrate community assembly. Oikos 2018, 127, 1586–1595. [Google Scholar] [CrossRef] [Green Version]
- Altermatt, F.; Bieger, A.; Carrara, F.; Rinaldo, A.; Holyoak, M. Effects of connectivity and recurrent local disturbances on community structure and population density in experimental metacommunities. PLoS ONE 2011, 6, e19525. [Google Scholar] [CrossRef]
- Levinsky, I.; Skov, F.; Svenning, J.-C.; Rahbek, C. Potential impacts of climate change on the distributions and diversity patterns of european mammals. Biodivers. Conserv. 2007, 16, 3803–3816. [Google Scholar] [CrossRef]
- Luo, Z.; Zhou, S.; Yu, W.; Yu, H.; Yang, J.; Tian, Y.; Zhao, M.; Wu, H. Impacts of climate change on the distribution of sichuan snub-nosed monkeys (Rhinopithecus roxellana) in shennongjia area, china. Am. J. Primatol. 2015, 77, 135–151. [Google Scholar] [CrossRef]






| Variable | Description | Contribution (%) |
|---|---|---|
| Bio13 | Precipitation of Wettest Month | 37.1 |
| Bio19 | Precipitation of Coldest Quarter | 17.9 |
| Bio4 | Temperature Seasonality | 14.3 |
| Bio17 | Precipitation of Driest Quarter | 10.7 |
| Bio7 | Temperature Annual Range | 10.4 |
| Bio10 | Mean Temperature of Warmest Quarter | 9.5 |
| Parameter | Description | Parameter Settings |
|---|---|---|
| rcThreshold | Habitat suitability data | 500 |
| envChgSteps | Number of the environmental change step | 5 |
| dispSteps | Number of the dispersal step | 20 |
| iniMatAge | Initial maturity age of newly colonized units | 1 |
| propaguleProd | Reproductive production potential of new colonization units over time | c(0.01 0.08 0.5 0.92) |
| lddFreq | the probability for an occupied cell to produce a long distance dispersal event | 0.1 |
| lddMinDist | Minimum distance for long distance dispersal | 6 |
| lddMaxDist | Maximum distance for long distance dispersal | 15 |
| replicateNb | Number of simulations repeated | 5 |
| Climate Scenario | Unlimited Dispersal | No Dispersal | Limited Dispersal |
|---|---|---|---|
| Initial | 4929 | 4929 | 4929 |
| SSP126 | 6524 | 75 | 319 |
| SSP245 | 5472 | 69 | 285 |
| SSP370 | 3026 | 0 | 2 |
| SSP585 | 1029 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-L.; Alvarez, F.; Whiting, M.J.; Qin, X.-D.; Chen, Z.-N.; Wu, Z.-J. Climate Change and Dispersal Ability Jointly Affects the Future Distribution of Crocodile Lizards. Animals 2022, 12, 2731. https://doi.org/10.3390/ani12202731
Zhang X-L, Alvarez F, Whiting MJ, Qin X-D, Chen Z-N, Wu Z-J. Climate Change and Dispersal Ability Jointly Affects the Future Distribution of Crocodile Lizards. Animals. 2022; 12(20):2731. https://doi.org/10.3390/ani12202731
Chicago/Turabian StyleZhang, Xiao-Li, Facundo Alvarez, Martin J. Whiting, Xu-Dong Qin, Ze-Ning Chen, and Zheng-Jun Wu. 2022. "Climate Change and Dispersal Ability Jointly Affects the Future Distribution of Crocodile Lizards" Animals 12, no. 20: 2731. https://doi.org/10.3390/ani12202731