Molecular Characterization of Lipoptena fortisetosa from Environmental Samples Collected in North-Eastern Poland
Abstract
:Simple Summary
Abstract
1. Introduction
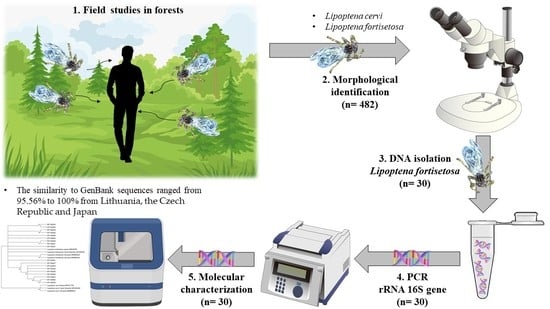
2. Materials and Methods
2.1. Sample Collection
2.2. Species Identification
2.3. Statistical Analysis
2.4. DNA Extraction
2.5. Polymerase Chain Reaction
2.6. Sequencing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schumann, H.; Messner, B. Erstnachweis von Lipoptena fortisetosa Maa, 1965 in Deutschland (Dipt., Hippoboscidae). Entomol. Nachr. Ber. 1993, 37, 247–249. [Google Scholar]
- Metelitsa, A.K.; Veselkin, G.A. Parasitism of the louse fly Lipoptena fortisetosa on cattle. Parazitologiia 1989, 23, 276–277. [Google Scholar] [PubMed]
- McAlpine, J.F. Manual of Nearctic Diptera; Research Branch, Agriculture Canada: Ottawa, ON, Canada, 1987; Volume 2, pp. 1271–1332. [Google Scholar]
- Gałęcki, R.; Jaroszewski, J.; Xuan, X.; Bakuła, T. Temporal-Microclimatic Factors Affect the Phenology of Lipoptena fortisetosa in Central European Forests. Animals 2020, 10, 2012. [Google Scholar] [CrossRef] [PubMed]
- Buczek, W.; Buczek, A.M.; Bartosik, K.; Buczek, A. Comparison of skin lesions caused by Ixodes ricinus ticks and Lipoptena cervi deer keds infesting humans in the natural environment. Int. J. Environ. Res. Public Health 2020, 17, 3316. [Google Scholar] [CrossRef] [PubMed]
- Madslien, K.; Ytrehus, B.; Vikøren, T.; Malmsten, J.; Isaksen, K.; Hygen, H.O.; Solberg, E.J. Hair-loss epizootic in moose (Alces alces) associated with massive deer ked (Lipoptena cervi) infestation. J. Wildl. Dis. 2011, 47, 893–906. [Google Scholar] [CrossRef]
- Maa, T.C. A synopsis of the Lipopteninae. J. Med. Entomol. 1965, 2, 233–248. [Google Scholar] [CrossRef]
- Kowal, J.; Nosal, P.; Kornaś, S.; Wajdzik, M.; Matysek, M.; Basiaga, M. Biodiversity and importance of hippoboscids infection in cervids. Med. Weter. 2016, 72, 745–749. [Google Scholar]
- Theodor, O. Lipoptena parvula, n. sp. eine neue Art aus der Tschechoslowakei (Diptera, Hippoboscidae). Acta Entomol. Mus. Nat. Pra. 1967, 37, 275–278. [Google Scholar]
- Zeman, P. Borrelia-infection rates in tick and insect vectors accompanying human risk of acquiring Lyme borreliosis in a highly endemic region in Central Europe. Folia Parasitol. 1998, 45, 319–325. [Google Scholar]
- Kurina, O.; Kirik, H.; Õunap, H.; Õunap, E. The northernmost record of a blood-sucking ectoparasite, Lipoptena fortisetosa Maa (Diptera: Hippoboscidae), in Estonia. Biodivers. Data J. 2019, 7, e47857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvetti, M.; Bianchi, A.; Marangi, M.; Barlaam, A.; Giacomelli, S.; Bertoletti, I.; Roy, L.; Giangaspero, A. Deer keds on wild ungulates in northern Italy, with a taxonomic key for the identification of spp. of Europe. Med. Vet. Entomol. 2020, 34, 74–85. [Google Scholar] [CrossRef]
- Andreani, A.; Sacchetti, P.; Belcari, A. Comparative morphology of the deer ked Lipoptena fortisetosa first recorded from Italy. Med. Vet. Entomol. 2019, 33, 140–153. [Google Scholar] [CrossRef] [Green Version]
- Oboňa, J.; Sychra, O.; Greš, S.; Heřman, P.; Manko, P.; Roháček, J.; Šestáková, A.; Šlapák, J.; Hromada, M. A revised annotated checklist of louse flies (Diptera, Hippoboscidae) from Slovakia. ZooKeys 2019, 862, 129–152. [Google Scholar] [CrossRef]
- Kadulski, S. Występowanie Stawonogów Pasożytniczych na Łownych Lagomorpha i Artiodactyla—Próba Syntezy; Rozprawy i Monografie 132; Wydawnictwo Uniwersytetu Gdańskiego: Gdańsk, Poland, 1989; pp. 1–140. [Google Scholar]
- Borowiec, L.; Zatwarnicki, T. Lipoptena fortisetosa Maa, 1965 (Diptera, Hippoboscidae), nowy gatunek dla fauny Polski. Prz. Zool. 1989, 33, 579–582. [Google Scholar]
- Kowal, J.; Nosal, P.; Rościszewska, M.; Matysek, M. New records of Lipoptena fortisetosa Maa, 1965 (Diptera:Hippoboscidae) in Poland. Dipteron 2009, 25, 27–29. [Google Scholar]
- Cydzik, K.; Kadulski, S. Parasitic Insects of the Red Deer (Cervus elaphus L.) in Northeastern Poland; Stawonogi. Inwazje i ich ograniczanie; Akapit: Lublin, Poland, 2009; pp. 113–115. [Google Scholar]
- Jędrysik, D.; Kadulski, S. Parasitic Arthropods of Roe Deer Capreolus capreolus (L.) of the Region of Pojezierze Południowobałtyckie (The Southern Baltic Lake District); Arthropods. The Medical and Economic Importance; Akapit: Lublin, Poland, 2012; pp. 95–103. [Google Scholar]
- Matysek, M.; Kowal, J. Dwa nowe gatunki muchówek. Tatry 2014, 48, 64–65. [Google Scholar]
- Sokół, R.; Gałęcki, R. Prevalence of keds on city dogs in central Poland. Med. Vet. Entomol. 2017, 31, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, T.; Werszko, J.; Steiner-Bogdaszewska, Ż.; Jeżewski, W.; Laskowski, Z.; Karbowiak, G. Molecular detection of Bartonella spp. in deer ked (Lipoptena cervi) in Poland. Parasit. Vectors 2017, 10, 487. [Google Scholar] [CrossRef] [Green Version]
- De Bruin, A.; Van Leeuwen, A.D.; Jahfari, S.; Takken, W.; Földvári, M.; Dremmel, L.; Sprong, H.; Földvári, G. Vertical transmission of Bartonella schoenbuchensis in Lipoptena cervi. Parasit. Vectors 2015, 8, 176. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, E.M.; Vera, C.P.; Pulliainen, A.T.; Sironen, T.; Aaltonen, K.; Kortet, R.; Härkönen, L.; Härkönen, S.; Paakkonen, T.; Nieminen, P.; et al. Molecular detection of Bartonella spp. in deer ked pupae, adult keds and moose blood in Finland. Epidemiol. Infect. 2015, 143, 578–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Kim, K.T.; Kwon, O.D.; Ock, Y.; Kim, T.; Choi, D.; Kwak, D. Novel detection of Coxiella spp., Theileria luwenshuni, and T. ovis endosymbionts in deer keds (Lipoptena fortisetosa). PLoS ONE 2016, 11, e0156727. [Google Scholar] [CrossRef] [Green Version]
- Doby, J.M.; Bigaignon, G.; Degeilh, B.; Guiguen, C. Ectoparasites of large wild mammals (deer and wild boars) and Lyme borreliosis. Search for Borrelia burgdorferi in more than 1400 ticks, lice, Pupipara Diptera and fleas. Rev. Med. Vet. 1994, 145, 743–748. [Google Scholar]
- Hulinsky, V.; Smetana, K. Molecular and microscopical evidence of Ehrlichia spp. and Borrelia burgdorferi sensu lato in patients, animals and ticks in the Czech Republic. Microbiologica 2002, 25, 437–448. [Google Scholar]
- Lane, R.S.; Mun, J.; Parker, J.M.; White, M. Columbian black-tailed deer (Odocoileus hemionus columbianus) as hosts for Borrelia spp. in northern California. J. Wildl. Dis. 2005, 41, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böse, R.; Petersen, K. Lipoptena cervi (Diptera), a potential vector of Megatrypanum trypanosomes of deer (Cervidae). Parasitol. Res. 1991, 77, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; De La Fuente, J.; Biró, N.; Fernández de Mera, I.G.; Meli, M.L.; Elek, V.; Gönczi, E.; Meili, T.; Tánczos, B.; Farkas, R.; et al. First molecular evidence of Anaplasma ovis and Rickettsia spp. in keds (Diptera: Hippoboscidae) of sheep and wild ruminants. Vector Borne Zoonotic Dis. 2011, 11, 1319–1321. [Google Scholar] [CrossRef] [Green Version]
- Víchová, B.; Majláthová, V.; Nováková, M.; Majláth, I.; Čurlík, J.; Bona, M.; Komjáti-Nagyová, M.; Peťko, B. PCR detection of re-emerging tick-borne pathogen, Anaplasma phagocytophilum, in deer ked (Lipoptena cervi) a blood-sucking ectoparasite of cervids. Biologia 2011, 66, 1082. [Google Scholar] [CrossRef]
- Gałęcki, R.; Jaroszewski, J.; Bakuła, T.; Galon, E.M.; Xuan, X. Molecular Detection of Selected Pathogens with Zoonotic Potential in Deer Keds (Lipoptena fortisetosa). Pathogens 2021, 10, 324. [Google Scholar] [CrossRef]
- Andreani, A.; Giangaspero, A.; Marangi, M.; Barlaam, A.; Ponzetta, M.P.; Roy, L.; Belcari, A.; Sacchetti, P. Asia and Europe: So Distant So Close? The Case of Lipoptena fortisetosa in Italy. Korean J. Parasitol. 2020, 58, 661–668. [Google Scholar] [CrossRef]
- Mysterud, A.; Madslien, K.; Herland, A.; Viljugrein, H.; Ytrehus, B. Phenology of deer ked (Lipoptena cervi) host-seeking flight activity and its relationship with prevailing autumn weather. Parasit. Vectors 2016, 9, 95. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, H.S.; Ewing, C.P. Comparison of flagging, walking, trapping, and collecting from hosts as sampling methods for northern deer ticks, Ixodes dammini, and lone-star ticks, Amblyomma americanum (Acari: Ixodidae). Exp. Appl. Acarol. 1989, 7, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Borowiec, L. Klucze do Oznaczania Owadów Polski; Część XXVIII. Muchówki-Diptera. Zeszyt 77, Wpleszczowate-Hippoboscidae; Państwowe Wydawnictwo Naukowe: Warsaw, Poland, 1984; pp. 3–35. [Google Scholar]
- Maa, T.C. A revised checklist and concise host index of Hippoboscidae (Diptera). Pac. Insects Monogr. 1969, 20, 261–299. [Google Scholar]
- Weinberger, R. Practical Capillary Electrophoresis; Academic Press: San Diego, CA, USA, 2000; pp. 423–457. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Molecular Evolutionary Genetics Analysis. Available online: https://www.megasoftware.net (accessed on 3 September 2019).
- Radzijevskaja, J.; Paulauskas, A.; Klepeckiene, K.; Razanske, I.; Rosef, O. Species Diversity and Molecular Characterization of Deer Keds (Genus Lipoptena) from Cervids. Unpublished. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MN889549 (accessed on 2 February 2020).
- Šochová, E.; Husník, F.; Nováková, E.; Halajian, A.; Hypša, V. Arsenophonus and Sodalis replacements shape evolution of symbiosis in louse flies. PeerJ 2017, 5, e4099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosokawa, T.; Nikoh, N.; Koga, R.; Satô, M.; Tanahashi, M.; Meng, X.Y.; Fukatsu, T. Reductive genome evolution, host–symbiont co-speciation and uterine transmission of endosymbiotic bacteria in bat flies. ISME J. 2012, 6, 577–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, A.G. The sex ratio in ectoparasitic insects. Ecol. Entomol. 1981, 6, 155–174. [Google Scholar] [CrossRef]
- Paakkonen, T. Ecophysiology of the Deer Ked (Lipoptena cervi) and Its Hosts. Ph.D. Thesis, University of Eastern Finland, Joensuu, Finland, 2012. Available online: https://core.ac.uk/download/pdf/15169155.pdf (accessed on 23 April 2020).
- Barrows, E.M. Animal Behavior Desk Reference: A Dictionary of Animal Behavior, Ecology, and Evolution; CRC Press: Boca Raton, FL, USA, 2011; p. 72. [Google Scholar]
- Kadulski, S. Ectoparasites of Cervidae in north-east Poland. Acta Parasitol. 1996, 41, 204–210. [Google Scholar]
- Szczurek, B.; Kadulski, S. Ectoparasites on fallow deer, Dama dama (L.) in Pomerania, Poland. Acta Parasitol. 2004, 49, 80–86. [Google Scholar]
- Vikøren, T.; Lillehaug, A.; Handeland, K. Helseovervakingsprogrammet for Hjortevilt (HOP); Årsrapport for 2006 og 2007, Report Series; National Veterinary Inst.: Oslo, Norway, 2008. [Google Scholar]
- Mihalca, A.D.; Păstrav, I.R.; Sándor, A.D.; Deak, G.; Gherman, C.M.; Sarmaşi, A.; Votýpka, J. First report of the dog louse fly Hippobosca longipennis in Romania. Med. Vet. Entomol. 2019, 33, 530–535. [Google Scholar] [CrossRef]
- Trout, R.T.; Steelman, C.D.; Szalanski, A.L. Phylogenetics and population genetics of the louse fly, Lipoptena mazamae, from Arkansas, USA. Med. Vet. Entomol. 2010, 24, 258–265. [Google Scholar] [PubMed]
- Szalanski, A.L.; Austin, J.W.; McKern, J.A.; Steelman, C.D.; Gold, R.E. Mitochondrial and ribosomal internal transcribed spacer (ITS1) diversity of the bed bug Cimex lectularius L. (Heteroptera: Cimicidae). J. Med. Entomol. 2008, 45, 229–236. [Google Scholar] [CrossRef]
- Krafsur, E.S.; Madsen, M.; Wohlford, D.L.; Mihok, S.; Griffiths, N.T. Population genetics of Glossina morsitans submorsitans (Diptera: Glossinidae). Bull. Entomol. Res. 2000, 90, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Kulakova, N.V.; Khasnatinov, M.A.; Sidorova, E.A.; Adel’shin, R.V.; Belikov, S.I. Molecular identification and phylogeny of Dermacentor nuttalli (Acari: Ixodidae). Parasitol. Res. 2014, 113, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Livanova, N.N.; Tikunov, A.Y.; Kurilshikov, A.M.; Livanov, S.G.; Fomenko, N.V.; Taranenko, D.E.; Kvashnina, A.E.; Tikunova, N.V. Genetic diversity of Ixodes pavlovskyi and I. persulcatus (Acari: Ixodidae) from the sympatric zone in the south of Western Siberia and Kazakhstan. Exp. Appl. Acarol. 2015, 67, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Peterson, F.T.; Meier, R.; Kutty, S.N.; Wiegmann, B.M. The phylogeny and evolution of host choice in the Hippoboscoidea (Diptera) as reconstructed using four molecular markers. Mol. Phylogenet. Evol. 2007, 45, 111–122. [Google Scholar] [CrossRef] [PubMed]


| Sampling Site | Lipoptena cervi | Lipoptena fortisetosa | Total | ||
|---|---|---|---|---|---|
| ♂ | ♀ | ♂ | ♀ | ||
| Ełk | 28 | 39 | 3 | 5 | 75 |
| Gołdap | 29 | 32 | 7 | 10 | 78 |
| Jedwabno | 34 | 33 | 6 | 8 | 81 |
| Kolno | 40 | 32 | 14 | 10 | 96 |
| Pieniężno | 20 | 27 | 7 | 7 | 61 |
| Ruciane-Nida | 38 | 33 | 9 | 11 | 91 |
| Sequence ID | Closest Match ID | Country of Origin | Percentage Match | Reference |
|---|---|---|---|---|
| MT182645 |
MF495939
MN889549 |
Czech Republic
Lithuania | 99.56% | Radzijevskaja et al. [41] Sochova et al. [42] |
| MT182646 | 99.78% | |||
| MT182647 | 98.68% | |||
| MT182648 | 99.58% | |||
| MT182649 | 99.36% | |||
| MT182650 | 99.58% | |||
| MT182651 | 100% | |||
| MT182652 | 98.32% | |||
| MT182653 | 99.04% | |||
| MT182654 | 98.93% | |||
| MT182655 | 98.93% | |||
| MT182656 | 98.91% | |||
| MT182657 | 98.43% | |||
| MT182658 | 99.36% | |||
| MT182659 | AB632589 | Japan | 95.56 % | Hosokawa et al. [43] |
| MT182660 |
MF495939
MN889549 |
Czech Republic
Lithuania | 99.36% | Radzijevskaja et al. [41] Sochova et al. [42] |
| MT182661 | 99.15% | |||
| MT182663 | 98.91% |
| Number of Lipoptena fortisetosa Specimens | ||||||
|---|---|---|---|---|---|---|
| Sampling Site | Ełk (n = 5) | Gołdap (n = 5) | Jedwabno (n = 5) | Kolno (n = 5) | Pieniężno (n = 5) | Ruciane-Nida (n = 5) |
| MT182645 | 2 | - | - | - | - | - |
| MT182646 | 1 | - | - | - | - | - |
| MT182647 | 1 | 1 | - | 1 | - | - |
| MT182648 | 1 | - | - | - | - | - |
| MT182649 | - | 1 | - | - | - | - |
| MT182650 | - | 1 | - | - | - | - |
| MT182651 | - | 1 | - | 1 | - | - |
| MT182652 | - | - | 1 | - | - | - |
| MT182653 | - | - | 1 | - | - | 2 |
| MT182654 | - | - | 1 | - | - | 1 |
| MT182655 | - | - | 1 | - | - | 1 |
| MT182656 | - | - | - | 1 | 1 | - |
| MT182657 | - | 1 | - | 1 | - | - |
| MT182658 | - | - | 1 | 1 | 1 | - |
| MT182659 | - | - | - | - | 1 | - |
| MT182660 | - | - | - | - | 1 | - |
| MT182661 | - | - | - | - | 1 | - |
| MT182663 | - | - | - | - | - | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gałęcki, R.; Xuan, X.; Bakuła, T.; Jaroszewski, J. Molecular Characterization of Lipoptena fortisetosa from Environmental Samples Collected in North-Eastern Poland. Animals 2021, 11, 1093. https://doi.org/10.3390/ani11041093
Gałęcki R, Xuan X, Bakuła T, Jaroszewski J. Molecular Characterization of Lipoptena fortisetosa from Environmental Samples Collected in North-Eastern Poland. Animals. 2021; 11(4):1093. https://doi.org/10.3390/ani11041093
Chicago/Turabian StyleGałęcki, Remigiusz, Xuenan Xuan, Tadeusz Bakuła, and Jerzy Jaroszewski. 2021. "Molecular Characterization of Lipoptena fortisetosa from Environmental Samples Collected in North-Eastern Poland" Animals 11, no. 4: 1093. https://doi.org/10.3390/ani11041093