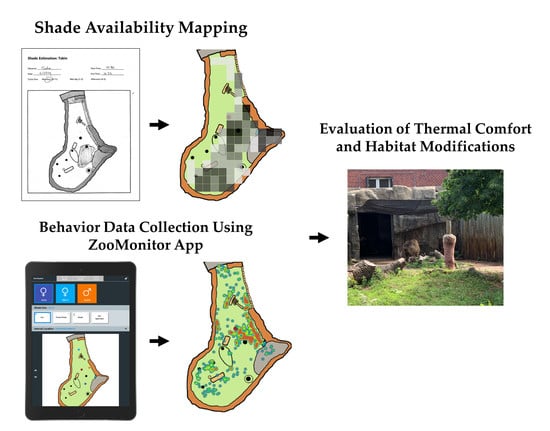
Mapping Shade Availability and Use in Zoo Environments: A Tool for Evaluating Thermal Comfort
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Part 1 Subjects: Outdoor Enclosures
2.2. Part 2 Subjects: Animals
2.3. Part 1 Data Collection: Shade Assessments
2.4. Part 2 Data Collection: Space Use Monitoring
2.5. Data Analysis
3. Results
3.1. Part 1: Assessing Zoo-Wide Shade Availability
3.2. Part 2: Case Study: Shade Use by a Group of Sichuan Takin
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuller, G.; Raghanti, M.A.; Dennis, P.M.; Kuhar, C.W.; Willis, M.A.; Schook, M.W.; Lukas, K.E. A comparison of nocturnal primate behavior in exhibits illuminated with red and blue light. Appl. Anim. Behav. Sci. 2016, 184, 126–134. [Google Scholar] [CrossRef]
- Orban, D.A.; Soltis, J.; Perkins, L.; Mellen, J.D. Sound at the zoo: Using animal monitoring, sound measurement, and noise reduction in zoo animal management. Zoo Biol. 2017, 36, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.R.; Gordon, T.A.; Eastcott, E.; Simpson, S.D.; Radford, A.N. Causes and consequences of intraspecific variation in animal responses to anthropogenic noise. Behav. Ecol. 2019, 30, 1501–1511. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, B.L. Making sense of it all: The importance of taking into account the sensory abilities of animals in their housing and management. Appl. Anim. Behav. Sci. 2018, 205, 175–180. [Google Scholar] [CrossRef]
- Martin, R.A.; Melfi, V. A comparison of zoo animal behavior in the presence of familiar and unfamiliar people. J. Appl. Anim. Sci. 2016, 19, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Woolway, E.E.; Goodenough, A.E. Effects of visitor numbers on captive European red squirrels (Sciurus vulgaris) and impacts on visitor experience. Zoo Biol. 2017, 36, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Chiew, S.J.; Butler, K.L.; Sherwen, S.L.; Coleman, G.J.; Fanson, K.V.; Hemsworth, P.H. Effects of regulating visitor viewing proximity and the intensity of visitor behaviour on little penguin (Eudyptula minor) behaviour and welfare. Animals 2019, 9, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, W.A., III. Scaling energetics of homeothermic vertebrates: An operational allometry. Ann. Rev. Phsyiol. 1987, 49, 107–120. [Google Scholar] [CrossRef]
- Veldhuis, M.P.; Kihwele, E.S.; Cromsigt, J.P.G.M.; Ogutu, J.O.; Hopcraft, J.G.C.; Owen-Smith, N.; Olff, H. Large herbivore assemblages in a changing climate: Incorporating water dependence and thermoregulation. Ecol. Lett. 2019, 22, 1536–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, A.; Mitchell, D.; Maloney, S.K.; Hetem, R.S. Towards a mechanistic understanding of the responses of large terrestrial mammals to heat and aridity associated with climate change. Clim. Chang. Res. 2016, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Czarick, M., III; Fairchild, B.D. Poultry housing in hot climates. In Poultry Production in Hot Climates, 2nd ed.; Daghir, N.J., Ed.; CABI: Oxfordshire, UK, 2008; pp. 80–131. [Google Scholar]
- Etches, R.J.; John, T.M.; Verrinder Gibbins, A.M. Behavioural, physiological, neuroendocrine and molecular responses to heat stress. In Poultry Production in Hot Climates, 2nd ed.; Daghir, N.J., Ed.; CABI: Oxfordshire, UK, 2008; pp. 80–131. [Google Scholar]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Polsky, L.; von Keyserlingk, M.A.G. Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, C.J. Thermal physiology of laboratory mice: Defining thermoneutrality. J. Therm. Biol. 2012, 37, 654–685. [Google Scholar] [CrossRef]
- Maloney, S.K.; Fuller, A.; Mitchell, D.; Gordon, C.; Overton, J.M. Translating animal model research: Does it matter that our rodents are cold? Physiology 2014, 29, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Katić, K.; Li, R.; Zeiler, W. Thermophysiological models and their applications: A review. Build. Environ. 2016, 106, 286–300. [Google Scholar] [CrossRef] [Green Version]
- IUPS Thermal Commission. Glossary of terms for thermal physiology: Third edition. Jpn. J. Physiol. 2001, 52, 245–280. [Google Scholar]
- Schrama, J.W.; van der Hel, W.; Gorssen, J.; Henken, A.M.; Verstegen, M.W.A.; Noordhuizen, J.P.T.M. Required thermal thresholds during transport of animals. Vet. Q. 1996, 18, 90–95. [Google Scholar] [CrossRef]
- Hill, R.A. Thermal constraints on activity scheduling and habitat choice in baboons. Am. J. Phys. Anthropol. 2006, 129, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Müller, E.F.; Kamau, J.M.Z.; Maloiy, G.M.O. A comparative study of basal metabolism and thermoregulation in a folivorous (Colobus guereza) and an omnivorous (Cercopithecus mitis) primate species. Comp. Biochem. Physiol. 1983, 74, 319–322. [Google Scholar] [CrossRef]
- Kingma, B.R.M.; Frijns, A.J.H.; Schellen, L.; van Marken Lichtenbelt, W.D. Beyond the classic thermoneutral zone. Temperature 2014, 1, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupp, R.F.; Vásquez, N.G.; Lamberts, R. A review of human thermal comfort in the built environment. Energy Build. 2015, 105, 178–205. [Google Scholar] [CrossRef]
- Fanger, P.O. Thermal Comfort: Analysis and Applications in Environmental Engineering; Danish Technical Press: Copenhagen, Denmark, 1970. [Google Scholar]
- Stevenson, R.D. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am. Nat. 1985, 126, 362–386. [Google Scholar] [CrossRef]
- Terrien, J.; Perret, M.; Aujard, F. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1428–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruetz, J.D. Evidence of cave use by savanna chimpanzees (Pan troglodytes verus) at Fongoli, Senegal: Implications for thermoregulatory behavior. Primates 2007, 48, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.B.; Rogers, A.R.; Schütz, K.E. Effect of solar radiation on dairy cattle behaviour, use of shade and body temperature in a pasture-based system. Appl. Anim. Behav. Sci. 2008, 109, 141–154. [Google Scholar] [CrossRef]
- Schütz, K.E.; Rogers, A.R.; Cox, N.R.; Tucker, C.B. Dairy cows prefer shade that offers greater protection against solar radiation in summer: Shade use, behaviour, and body temperature. Appl. Anim. Behav. Sci. 2009, 116, 28–34. [Google Scholar] [CrossRef]
- Coimbra, P.A.D.; Filho, L.C.P.M.; Hötzel, M.J. Effects of social dominance, water trough location and shade availability on drinking behaviour of cows on pasture. Appl. Anim. Behav. Sci. 2012, 139, 175–182. [Google Scholar] [CrossRef]
- McMillan, F.D. The mental health and well-being of personal control in animals. In Mental Health and Well-Being in Animals, 2nd ed.; Franklin D. McMillan, Ed.; CABI: Ames, IA, USA, 2005; pp. 67–81. [Google Scholar]
- Langman, V.A.; Rowe, M.; Forthman, D.; Whitton, B.; Langman, N.; Roberts, T.; Huston, K.; Boling, C.; Maloney, D. Thermal assessment of zoological exhibits I: Sea lion enclosure at the Audubon Zoo. Zoo Biol. 1996, 15, 403–411. [Google Scholar] [CrossRef]
- Langman, V.A.; Rowe, M.; Forthman, D.; Langman, N.; Black, J.; Walker, T. Quantifying shade using a standard environment. Zoo Biol. 2003, 22, 253–260. [Google Scholar] [CrossRef]
- Wark, J.D.; Kuhar, C.W.; Lukas, K.E. Behavioral thermoregulation in a group of zoo-housed colobus monkeys (Colobus guereza). Zoo Biol. 2014, 33, 257–266. [Google Scholar] [CrossRef]
- Pixlr; Inmagine: Chicago, IL, USA, 2020; Available online: http://pixlr.com/e (accessed on 9 July 2020).
- Zeller, U.; Göttert, T. The relations between evolution and domestication reconsidered—Implications for systematics, ecology, and nature conservation. Glob. Ecol. Conserv. 2019, 20, e00756. [Google Scholar] [CrossRef]
- ZooMonitor, version 3.2; Lincoln Park Zoo: Chicago, IL, USA, 2020; Available online: https://zoomnitor.org (accessed on 9 July 2020).
- Martin, P.; Bateson, P. Measuring Behavior: An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Wark, J.D.; Cronin, K.A.; Niemann, T.; Shender, M.A.; Horrigan, A.; Kao, A.; Ross, M.R. Monitoring the behavior and habitat use of animals to enhance welfare using the ZooMonitor app. Anim. Behav. Cogn. 2019, 6, 158–167. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 June 2020).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-148. 2020. Available online: https://CRAN.R-project.org/package=nlme (accessed on 1 June 2020).
- Zeng, Z.-G.; Beck, P.S.A.; Wang, T.-J.; Skidmore, A.K.; Song, Y.-L.; Gong, H.-S.; Prins, H.H.T. Effects of plant phenology and solar radiation on seasonal movement of golden takin in the Quinling Mountains, China. J. Mammal. 2010, 91, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.; Yang, S.; Leng, J.; Xu, S.; Tang, S.; Liu, C.; Gao, Y.; Mao, H. Impacts of shade on physiological and behavioural pattern of Dehong buffalo calves under high temperature. Appl. Anim. Behav. Sci. 2016, 177, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Schütz, K.E.; Cox, N.R.; Matthews, L.R. How important is shade to dairy cattle? Choice between shade or lying following different levels of lying deprivation. Appl. Anim. Behav. Sci. 2008, 114, 307–318. [Google Scholar] [CrossRef]
- Shrestha, A.K.; van Wieren, S.E.; van Langevelde, F.; Fuller, A.; Hetem, R.S.; Meyer, L.; de Bie, S.; Prins, H.H.T. Larger antelopes are sensitive to heat stress throughout all seasons but smaller antelopes only during summer in an African semi-arid environment. Int. J. Biometeorol. 2014, 58, 41–49. [Google Scholar] [CrossRef]
- du Toit, J.T.; Yetman, C.A. Effects of body size on the diurnal activity budgets of African browsing ruminants. Oecologia 2005, 143, 317–325. [Google Scholar] [CrossRef]
- Aublet, J.F.; Festa-Bianchet, M.; Bergero, D.; Bassano, B. Temperature constraints on foraging behaviour of male Alpine ibex (Capra ibex) in summer. Oecologia 2009, 159, 237–247. [Google Scholar] [CrossRef]
- Owen-Smith, N.; Goodall, V. Coping with savanna seasonality: Comparative daily activity patterns of African ungulates as revealed by GPS telemetry. J. Zool. 2014, 293, 181–191. [Google Scholar] [CrossRef]
- Powell, D.; Speeg, B.; Li, S.; Blumer, E.; McShea, W. An ethogram and activity budget of captive Sichuan takin (Budorcas taxicolor tibetana) with comparisons to other Bovidae. Mammalia 2013, 77, 391–401. [Google Scholar] [CrossRef]






| Enclosure Name | Species | No. of Animals | Enclosure Size (m2) |
|---|---|---|---|
| Alpaca | Alpaca (Vicugna vicugna f. pacos) | 1 | 554.4 |
| Camel | Bactrian Camel (Camelus bactrianus) | 3 | 1034.66 |
| Camel/Zebra | Bactrian Camel (C. bactrianus), Grevy’s Zebra (Equus grevyi) | 1, 1 | 652.47 |
| Chicken | Domestic Chicken (Gallus gallus f. domestica) | 15 | 87.77 |
| Cow | Domestic Cattle (Bos primigenius f. taurus) | 3 | 584.23 |
| Cow and Goat * | Domestic Cattle (B. primigenius f. taurus), Domestic Goat (Capra aegagrus f. hircus) | 1, 1 | 295.19 |
| Goat | Domestic Goat (C. aegagrus f. hircus) | 7 | 94.7 |
| Kangaroo | Red Kangaroo (Macropus rufus) | 3 | 378.67 |
| Lion 2 | African Lion (Panthera leo) | 3 | 632.99 |
| Lion 1 | African Lion (P. leo) | 3 | 657.1 |
| Ostrich | Ostrich (Struthio camelus) | 2 | 664.75 |
| Peccary | Chacoan Peccary (Catagonus wagneri) | 3 | 458.83 |
| Penguin | African Penguin (Spheniscus demersus) | 17 | 82.23 |
| Polar Bear | Polar Bear (Ursus maritimus) | 2 | 637.82 |
| Pony | Pony (Equus ferus f. caballus) | 3 | 500.3 |
| Puma | Puma (Puma concolor) | 1 | 199.48 |
| Rabbit | Flemish Giant Rabbit (Oryctolagus cuniculus f. domestica) | 2 | 21.25 |
| Gorilla | Western Lowland Gorilla (Gorilla gorilla gorilla) | 5, 4–7 | 1001.73 |
| Chimp/Gorilla 1 | Chimpanzee (Pan. troglodytes), Western Lowland Gorilla (G. gorilla gorilla) | 5, 4–7 | 453.74 |
| Chimp/Gorilla 2 | Chimpanzee (P. troglodytes), Western Lowland Gorilla (G. gorilla gorilla) | 5, 4–7 | 614.05 |
| Rhino 1 and 2 | Eastern Black Rhinoceros (Diceros bicornis michaeli) | 2 | 1140.38 |
| Rhino 3 | Eastern Black Rhinoceros (D. bicornis michaeli) | 1 | 492.37 |
| Red River Hog | Red River Hog (Potamochoerus porcus) | 2 | 265.86 |
| Giraffe and Zebra * | Giraffe (Giraffa camelopardalis rothschildi), Plains Zebra (Equus quagga) | 2, 2 | 1112.41 |
| Seal * | Grey Seal (Halichoerus grypus), Harbor Seal (Phoca vitulina concolor) | 1, 1 | 459.83 |
| Snow Leopard | Snow Leopard (Panthera uncia) | 1 | 190.22 |
| Snowy Owl | Snowy Owl (Budo scandiacus) | 2 | 78.74 |
| Takin 1 | Sichuan Takin (Budorcas taxicolor tibetana) | 3 | 504.88 |
| Takin 2 | Sichuan Takin (B. taxicolor tibetana) | 1 | 542.88 |
| Vulture and Stork * | Cinereous Vulture (Aegypius monachus), White Stork (Ciconia ciconia) | 2, 2 | 291.41 |
| Painted Dog | African Painted Dog (Lycaon pictus) | 2 | 347.21 |
| Zebra 1 | Grevy’s Zebra (E. grevyi) | 3 | 1551.65 |
| Zebra 2 | Grevy’s Zebra (E. grevyi) | 1 | 881.44 |
| Predictor | Estimate | CI | SE | p | |
|---|---|---|---|---|---|
| Intercept | 32.93 | 27.72 | 38.14 | 2.6 | <0.0001 |
| Season: Summer | 18.78 | 12.80 | 24.77 | 3.0 | <0.0001 |
| Season: Fall | 28.26 | 22.28 | 34.25 | 3.0 | <0.0001 |
| Time of Day: Midday | −6.76 | −12.74 | −0.78 | 3.0 | 0.027 |
| Time of Day: Afternoon | 6.42 | 0.43 | 12.40 | 3.0 | 0.036 |
| Summer × Midday | −0.98 | −9.44 | 7.48 | 4.3 | 0.82 |
| Fall × Midday | 2.95 | −5.51 | 11.42 | 4.3 | 0.49 |
| Summer × Afternoon | 2.21 | −6.26 | 10.67 | 4.3 | 0.61 |
| Fall × Afternoon | 16.66 | 8.20 | 25.12 | 4.3 | 0.0001 |
| Season | Time Period | Mean | Min | Max | Range | SD |
|---|---|---|---|---|---|---|
| Spring | Overall | 32.8 | 17.4 | 47.2 | 29.9 | 8.45 |
| Morning | 32.9 | 7.14 | 59.4 | 52.3 | 12.2 | |
| Midday | 26.2 | 6.86 | 44.6 | 37.7 | 9.61 | |
| Afternoon | 39.3 | 15.5 | 63.8 | 48.2 | 12.4 | |
| Summer | Overall | 52.0 | 22.8 | 84.9 | 62.2 | 16.2 |
| Morning | 51.7 | 14.9 | 86.7 | 71.8 | 19.9 | |
| Midday | 44.0 | 12.5 | 84.9 | 72.4 | 17.6 | |
| Afternoon | 60.3 | 32.5 | 91.5 | 59.0 | 17.6 | |
| Fall | Overall | 67.6 | 42.4 | 94.3 | 52.0 | 11.4 |
| Morning | 61.2 | 12.5 | 90.9 | 78.4 | 19.4 | |
| Midday | 57.4 | 25.4 | 96.4 | 71.0 | 15.2 | |
| Afternoon | 84.3 | 67.2 | 99.0 | 31.8 | 7.67 |
| Model | Coefficient | Estimate | SE | z | p |
|---|---|---|---|---|---|
| Female 1 | Intercept | −2.44 | 0.30 | −8.15 | <0.001 |
| Temperature | 0.21 | 0.021 | 9.72 | <0.001 | |
| Female 2 | Intercept | −2.25 | 0.28 | −7.98 | <0.001 |
| Temperature | 0.19 | 0.019 | 9.81 | <0.001 | |
| Male | Intercept | −1.42 | 0.26 | −5.56 | <0.001 |
| Temperature | 0.21 | 0.027 | 8.24 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wark, J.D.; Wierzal, N.K.; Cronin, K.A. Mapping Shade Availability and Use in Zoo Environments: A Tool for Evaluating Thermal Comfort. Animals 2020, 10, 1189. https://doi.org/10.3390/ani10071189
Wark JD, Wierzal NK, Cronin KA. Mapping Shade Availability and Use in Zoo Environments: A Tool for Evaluating Thermal Comfort. Animals. 2020; 10(7):1189. https://doi.org/10.3390/ani10071189
Chicago/Turabian StyleWark, Jason D., Natasha K. Wierzal, and Katherine A. Cronin. 2020. "Mapping Shade Availability and Use in Zoo Environments: A Tool for Evaluating Thermal Comfort" Animals 10, no. 7: 1189. https://doi.org/10.3390/ani10071189