Development of Lactic Acid-Fermented Tomato Products
Abstract
:1. Introduction
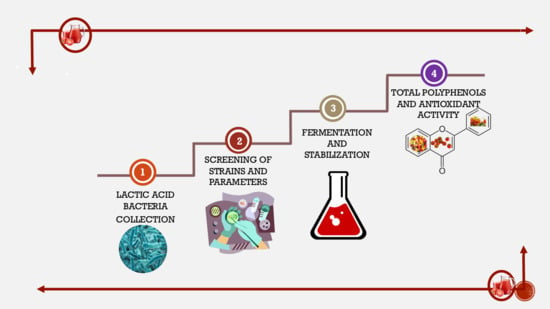
2. Materials and Methods
2.1. Tomato-Based Substrates
2.2. Lactic Acid Bacteria Strains
2.3. Experimental Plan for the Selection of Fermentation Parameters
2.4. Fermentation Process
2.5. Measurements of Antioxidant Activity
2.6. Evaluation of Total Phenolic Content
2.7. Statistical Analysis
3. Results and Discussion
3.1. Microbial Growth
3.2. Nutritional Features
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Ascherio, A.; Giovannucci, E.; Spiegelman, D.; Stampfer, M.J.; Willett, W.C.; Rimm, N.; Giovannucci, M. Vegetable, Fruit, and Cereal Fiber Intake and Risk of Coronary Heart Disease Among Men. JAMA 1996, 275, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Joshipura, K.J.; Ascherio, A.; Manson, J.A.E.; Stampfer, M.J.; Rimm, E.B.; Speizer, F.E.; Hennekens, C.H.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake in relation to risk of ischemic stroke. J. Am. Med. Assoc. 1999, 282, 1233–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welman, A.D. Exploitation of exopolysaccharides from lactic acid bacteria. In Bacterial Polysaccharides: Current Innovations and Future Trends; Ullrich, M., Ed.; Caister Academic Press; School of Engineering and Science, Jacobs University Bremen: Bremen, Germany, 2009; pp. 331–344. ISBN 978-1-904455-45-5. [Google Scholar]
- Holzapfel, W.H.; Wood, B.B.J. Introduction to the LAB. In Lactic Acid Bacteria: Biodiversity and Taxonomy; John Wiley & Sons, Ltd.: West Sussex, UK, 2014; pp. 1–12. [Google Scholar]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Calasso, M.; Gobbetti, M. Proteomics of the bacterial cross-talk by quorum sensing. J. Proteom. 2011, 74, 19–34. [Google Scholar] [CrossRef]
- Espirito-Santo, A.P.; Carlin, F.; Renard, C.M.G.C. Apple, grape or orange juice: Which one offers the best substrate for lactobacilli growth?—A screening study on bacteria viability, superoxide dismutase activity, folates production and hedonic characteristics. Food Res. Int. 2015, 78, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; De Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [Green Version]
- Filannino, P.; Cardinali, G.; Rizzello, C.G.; Buchin, S.; De Angelis, M.; Gobbetti, M.; Di Cagno, R. Metabolic responses of Lactobacillus plantarum strains during fermentation and storage of vegetable and fruit juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215. [Google Scholar] [CrossRef] [Green Version]
- Ricci, A.; Cirlini, M.; Maoloni, A.; Del Rio, D.; Calani, L.; Bernini, V.; Galaverna, G.; Neviani, E.; Lazzi, C. Use of dairy and plant-derived lactobacilli as starters for cherry juice fermentation. Nutrients 2019, 11, 213. [Google Scholar] [CrossRef] [Green Version]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Dorais, M.; Ehret, D.L.; Papadopoulos, A.P. Tomato (Solanum lycopersicum) health components: From the seed to the consumer. Phytochem. Rev. 2008, 7, 231–250. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rissanen, T.H.; Voutilainen, S.; Nyyssönen, K.; Lakka, T.A.; Sivenius, J.; Salonen, R.; Kaplan, G.A.; Salonen, J.T. Low serum lycopene concentration is associated with an excess incidence of acute coronary events and stroke: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2001, 85, 749–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sesso, H.D.; Liu, S.; Gaziano, J.M.; Buring, J.E. Dietary Lycopene, Tomato-Based Food Products and Cardiovascular Disease in Women. J. Nutr. 2003, 133, 2336–2341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, A.; Bernini, V.; Maoloni, A.; Cirlini, M.; Galaverna, G.; Neviani, E.; Lazzi, C. Vegetable by-product lacto-fermentation as a new source of antimicrobial compounds. Microorganisms 2019, 7, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventòs, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzym. 1999, 14, 152–178. [Google Scholar] [CrossRef]
- Ricci, A.; Levante, A.; Cirlini, M.; Calani, L.; Bernini, V.; Del Rio, D.; Galaverna, G.; Neviani, E.; Lazzi, C. The influence of viable cells and cell-free extracts of lactobacillus casei on volatile compounds and polyphenolic profile of elderberry juice. Front. Microbiol. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Cirlini, M.; Ricci, A.; Galaverna, G.; Lazzi, C. Application of lactic acid fermentation to elderberry juice: Changes in acidic and glucidic fractions. LWT Food Sci. Technol. 2020, 118, 1–6. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Calani, L.; Bernini, V.; Neviani, E.; Del Rio, D.; Galaverna, G.; Lazzi, C. In vitro metabolism of elderberry juice polyphenols by lactic acid bacteria. Food Chem. 2019, 276, 692–699. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Levante, A.; Dall’Asta, C.; Galaverna, G.; Lazzi, C. Volatile profile of elderberry juice: Effect of lactic acid fermentation using L. plantarum, L. rhamnosus and L. casei strains. Food Res. Int. 2018, 105, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Surico, R.F.; Paradiso, A.; De Angelis, M.; Salmon, J.C.; Buchin, S.; De Gara, L.; Gobbetti, M. Effect of autochthonous lactic acid bacteria starters on health-promoting and sensory properties of tomato juices. Int. J. Food Microbiol. 2009, 128, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.H.; Kim, Y.; Oh, J.H. Chemical characterization of tomato juice fermented with bifidobacteria. J. Food Sci. 2010, 75, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Juven, B.J.; Weisslowicz, H. Chemical Changes in Tomato Juices Caused by Lactic Acid Bacteria. J. Food Sci. 1981, 46, 1543–1545. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, H.P.; Grover, J. Fermentation of Tomato juice by Probiotic Lactic acid bacteria. Int. J. Adv. Pharm. Biol. Chem. 2016, 5, 212–219. [Google Scholar]
- Viskelis, P.; Juodeikiene, G.; Urbonaviciene, D.; Bartkiene, E.; Vidmantiene, D. Lactic Acid Fermentation of Tomato: Effects on cis/trans Lycopene Isomer Ratio, β-Carotene Mass Fraction and Formation of L(+)- and D(–)-Lactic Acid. Food Technol. Biotechnol. 2013, 51, 471–478. [Google Scholar]
- Liu, Y.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, G.; Chen, W. Beneficial Effects of Tomato Juice Fermented by Lactobacillus Plantarum and Lactobacillus Casei: Antioxidation, Antimicrobial Effect, and Volatile Profiles. Molecules 2018, 23, 2366. [Google Scholar] [CrossRef] [Green Version]
- Nursiwi, A.; Nurhartadi, E.; Utami, R.; Sari, A.M.; Laksono, P.W.; Aprilia, E.N. Characteristic of Fermented Whey Beverage with Addition of Tomato Juice (Lycopersicum esculentum). IOP Conf. Ser. Mater. Sci. Eng. 2017, 193, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Ma, C.; Gong, G.; Chang, C. Fermentation parameters, antioxidant capacities, and volatile flavor compounds of tomato juice-skim milk mixtures fermented by Lactobacillus plantarum ST-III. Food Sci. Biotechnol. 2019, 28, 1147–1154. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Manzocco, L. Oil stability and antioxidant properties of an oil tomato food system as affected by processing. Adv. Food Sci. 1999, 21, 10–14. [Google Scholar]
- Van Het Hof, K.H.; de Boer, B.C.J.; Tijburg, L.B.; Lucius, B.R.; Zijp, I.; West, C.E.; Hautvast, J.G.A.J.; Weststrate, J.A. Carotenoid Bioavailability in Humans from Tomatoes Processed in Different Ways Determined from the Carotenoid Response in the Triglyceride-Rich Lipoprotein Fraction of Plasma after a Single Consumption and in Plasma after Four Days of Consumption. J. Nutr. 2000, 130, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Hervert-Hernández, D.; Pintado, C.; Rotger, R.; Goñi, I. Stimulatory role of grape pomace polyphenols on Lactobacillus acidophilus growth. Int. J. Food Microbiol. 2009, 136, 119–122. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, A.; Bartolomé, B.; Martínez-Rodríguez, A.J.; Pueyo, E.; Martín-Alvarez, P.J.; Moreno-Arribas, M. V Potential of phenolic compounds for controlling lactic acid bacteria growth in wine. Food Control 2008, 19, 835–841. [Google Scholar] [CrossRef]






| CA | CG | CV |
|---|---|---|
| Orange tomato juice 28%, Williams pear puree 22.3%, white grape juice 16%, carrot juice 14.2%, orange juice 13.8%, ginger puree 0.8% | Yellow tomato juice 27%, Golden Delicious apple puree 24%, white grape juice 24%, Williams pear puree 10%, yellow carrot juice 10%, lemon juice 0.1% | Tomato juice 27%, strawberry puree 14.6%, pomegranate juice 14.6%, white grape juice 14.6%, Golden Delicious apple puree 14.6%, purple carrot juice 13.6% |
| AA (mmolTEAC/mL) | P (mgGAE/L) | ||||
|---|---|---|---|---|---|
| NTT | TT | NTT | TT | ||
| CA | NF | 1.23 ± 0.02 | 347.90 ± 1.47 | ||
| 1 | 1.21 ± 0.02 | 1.20 ± 0.02 | 386.38 ± 2.59 * | 335.62 ± 0.72 * | |
| 2 | 1.21 ± 0.02 | 1.27 ± 0.02 | 396.95 ± 0.33 * | 332.00 ± 0.76 * | |
| 3 | 1.37 ± 0.02 * | 1.33 ± 0.02 * | 352.19 ± 0.44 * | 356.19 ± 0.44 * | |
| 4 | 1.12 ± 0.02 * | 1.18 ± 0.03 | 330.00 ± 0.76 * | 342.67 ± 0.16 * | |
| 5 | 1.26 ± 0.03 | 1.30 ± 0.01 * | 436.57 ± 0.57 * | 337.43 ± 0.49 * | |
| 6 | 1.29 ± 0.02 | 1.25 ± 0.01 | 347.52 ± 1.94 | 292.95 ± 2.40 * | |
| 7 | 1.18 ± 0.01 * | 1.37 ± 0.02 * | 325.33 ± 2.03 * | 343.81 ± 1.15 * | |
| 8 | 1.28 ± 0.01 * | 1.16 ± 0.01 * | 326.38 ± 0.66 * | 346.57 ± 6.96 | |
| CG | NF | 2.12 ± 0.02 | 324.57 ± 1.51 | ||
| 1 | 2.20 ± 0.03 * | 2.79 ± 0.04 * | 435.71 ± 2.00 * | 333.71 ± 1.31 * | |
| 2 | 2.49 ± 0.25 * | 2.51 ± 0.02 | 380.48 ± 0.33 * | 336.29 ± 0.49 * | |
| 3 | 2.62 ± 002 * | 2.42 ± 0.02 * | 480.29 ± 2.29 * | 343.14 ± 2.27 * | |
| 4 | 2.50 ± 0.01 * | 2.30 ± 0.06 * | 352.48 ± 1.41 * | 318.38 ± 0.59 * | |
| 5 | 2.37 ± 0.02 * | 2.63 ± 0.02 * | 381.33 ± 0.66 * | 411.43 ± 0.29 * | |
| 6 | 2.87 ± 0.10 * | 2.60 ± 0.06 * | 297.24 ± 3.00 * | 297.90 ± 3.06 * | |
| 7 | 2.73 ± 0.08 * | 2.96 ± 0.03 * | 319.62 ± 0.59 * | 361.33 ± 6.58 * | |
| 8 | 2.60 ± 0.01 * | 2.90 ± 0.11 * | 404.67 ± 1.94 * | 319.62 ± 2.50 | |
| CV | NF | 17.10 ± 0.07 | 1366.32 ± 17.31 | ||
| 1 | 17.05 ± 0.19 | 17.23 ± 0.09 | 1202.22 ± 2.69 * | 1173.52 ± 3.38 * | |
| 2 | 16.32 ± 0.08 * | 16.65 ± 0.10 * | 1195.33 ± 0.67 * | 1288.81 ± 1.72 * | |
| 3 | 16.44 ± 0.11 * | 16.18 ± 0.14 * | 1038.89 ± 1.39 * | 1195.43 ± 0.40 * | |
| 4 | 16.60 ± 0.18 * | 16.84 ± 0.14 | 1231.78 ± 0.77 * | 1321.92 ± 0.68 * | |
| 5 | 16.16 ± 0.13 * | 15.26 ± 0.01 * | 1086.44 ± 0.38 * | 1137.67 ± 0.68 * | |
| 6 | 16.89 ± 0.13 | 11.16 ± 0.09 * | 1264.89 ± 0.38 * | 1154.79 ± 0.68 * | |
| 7 | 17.23 ± 0.17 | 15.91 ± 0.15 * | 1316.44 ± 0.38 * | 1157.31 ± 0.40 * | |
| 8 | 18.37 ± 0.15 * | 11.84 ± 0.17 * | 1428.44 ± 1.92 * | 1412.33 ± 0.01 * | |
| C | NF | 7.21 ± 0.08 | 2354.89 ± 4.56 | ||
| 1 | 5.21 ± 0.05 * | 4.72 ± 0.16 * | 1392.33 ± 1.10 * | 1319.82 ± 4.11 * | |
| 2 | 5.26 ± 0.14 * | 5.66 ± 0.21 * | 1306.48 ± 0.84 * | 1500.09 ± 0.84 * | |
| 3 | 6.61 ± 0.01 * | 6.31 ± 0.12 * | 1553.61 ± 3.57 * | 1490.59 ± 0.32 * | |
| 4 | 5.00 ± 0.15 * | 5.84 ± 0.08 * | 1567.31 ± 3.85 * | 1884.20 ± 0.63 * | |
| 5 | 6.46 ± 0.05 * | 6.05 ± 0.14 * | 1564.20 ± 3.65 * | 1482.01 ± 5.62 * | |
| 6 | 5.80 ± 0.09 * | 5.78 ± 0.11 * | 1531.32 ± 1.14 * | 1451.51 ± 5.28 * | |
| 7 | 6.10 ± 0.09 * | 5.94 ± 0.08 * | 1502.47 ± 4.68 * | 1461.37 ± 0.55 * | |
| 8 | 6.28 ± 0.04 * | 5.72 ± 0.07 * | 1433.42 ± 1.45 * | 1430.14 ± 1.98 * | |
| SPC | NF | 1.59 ± 0.13 | 263.14 ± 0.43 | ||
| 1 | 2.20 ± 0.36 | 1.65 ± 0.08 | 256.48 ± 4.30 | 239.00 ± 0.14 * | |
| 2 | 1.59 ± 0.05 | 2.71 ± 0.23 * | 307.52 ± 0.82 * | 201.19 ± 0.22 * | |
| 3 | 2.44 ± 0.05 * | 2.36 ± 0.25 * | 228.48 ± 0.16 * | 212.24 ± 0.22 * | |
| 4 | 1.90 ± 0.18 | 1.23 ± 0.03 * | 319.62 ± 0.87 * | 307.90 ± 0.33 * | |
| 5 | 2.65 ± 0.01 * | 3.27 ± 0.49 * | 294.76 ± 1.29 * | 298.29 ± 0.89 * | |
| 6 | 3.11 ± 0.08 * | 2.90 ± 0.17 * | 253.71 ± 2.00 * | 337.48 ± 0.08 * | |
| 7 | 2.46 ± 0.08 * | 2.59 ± 0.16 * | 281.05 ± 4.52 * | 254.62 ± 0.50 * | |
| 8 | 2.67 ± 0.17 * | 3.38 ± 0.09 * | 284.48 ± 0.16 * | 302.33 ± 0.08 * | |
| SPP | NF | 2.38 ± 0.06 | 265.81 ± 0.59 | ||
| 1 | 3.70 ± 0.19 * | 2.39 ± 0.19 | 384.19 ± 5.75 * | 265.14 ± 0.29 | |
| 2 | 2.88 ± 0.20 | 2.21 ± 0.21 | 209.90 ± 0.16 * | 185.43 ± 0.01 * | |
| 3 | 2.73 ± 0.01 * | 2.09 ± 0.05 * | 224.95 ± 1.29 * | 247.05 ± 0.16 * | |
| 4 | 3.13 ± 0.12 * | 3.13 ± 0.12 * | 264.57 ± 0.01 | 264.57 ± 0.01 | |
| 5 | 3.16 ± 0.10 * | 3.17 ± 0.07 * | 382.19 ± 0.44 * | 187.71 ± 0.76 * | |
| 6 | 3.04 ± 0.19 * | 2.11 ± 0.18 | 290.57 ± 0.29 * | 239.62 ± 0.33 * | |
| 7 | 3.11 ± 0.02 * | 2.48 ± 0.10 | 379.62 ± 0.92 * | 234.10 ± 0.33 * | |
| 8 | 2.30 ± 0.11 | 2.64 ± 0.07 * | 367.62 ± 0.17 * | 365.43 ± 0.86 * | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, A.; Marrella, M.; Hadj Saadoun, J.; Bernini, V.; Godani, F.; Dameno, F.; Neviani, E.; Lazzi, C. Development of Lactic Acid-Fermented Tomato Products. Microorganisms 2020, 8, 1192. https://doi.org/10.3390/microorganisms8081192
Ricci A, Marrella M, Hadj Saadoun J, Bernini V, Godani F, Dameno F, Neviani E, Lazzi C. Development of Lactic Acid-Fermented Tomato Products. Microorganisms. 2020; 8(8):1192. https://doi.org/10.3390/microorganisms8081192
Chicago/Turabian StyleRicci, Annalisa, Martina Marrella, Jasmine Hadj Saadoun, Valentina Bernini, Francesco Godani, Franco Dameno, Erasmo Neviani, and Camilla Lazzi. 2020. "Development of Lactic Acid-Fermented Tomato Products" Microorganisms 8, no. 8: 1192. https://doi.org/10.3390/microorganisms8081192