Application of Biopsy Samples Used for Helicobacter pylori Urease Test to Predict Epstein–Barr Virus-Associated Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Materials
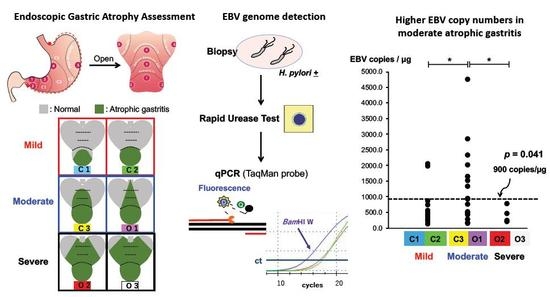
2.2. Atrophic Gastritis Diagnosis
2.3. H. Pylori Detection by RUT
2.4. gDNA Preparation
2.5. qPCR Targeting EBV gDNA
2.6. Statistical Analysis
3. Results
3.1. Detection of EBV gDNA Using qPCR
3.2. EBV gDNA Load in Atrophic Gastritis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hatakeyama, M. Helicobacter pylori CagA and gastric cancer: A paradigm for hit-and-run carcinogenesis. Cell Host Microbe 2014, 15, 306–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Jha, H.C. Status of Epstein-Barr virus coinfection with Helicobacter pylori in gastric cancer. J. Oncol. 2017, 2017, 3456264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iizasa, H.; Nanbo, A.; Nishikawa, J.; Jinushi, M.; Yoshiyama, H. Epstein-Barr virus (EBV)-associated gastric carcinoma. Viruses 2012, 4, 3420–3439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saju, P.; Murata-Kamiya, N.; Hayashi, T.; Senda, Y.; Nagase, L.; Noda, S.; Matsusaka, K.; Funata, S.; Kunita, A.; Urabe, M.; et al. Host SHP1 phosphatase antagonizes Helicobacter pylori CagA and can be downregulated by Epstein-Barr virus. Nat. Microbiol. 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Pandey, S.; Jha, H.C.; Shukla, S.K.; Shirley, M.K.; Robertson, E.S. Epigenetic regulation of tumor suppressors by Helicobacter pylori enhances EBV-induced proliferation of gastric epithelial cells. mBio 2018, 9, e00649-18. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, J.; Shuto, T.; Yanagi, A.; Takagi, T.; Ogawa, R.; Sasaki, S.; Goto, A.; Hamabe, K.; Hashimoto, S.; Okamoto, T.; et al. Epstein-Barr virus-associated gastric carcinomas developed after successful eradication of Helicobacter pylori. Clin. J. Gastroenterol. 2020, 1–6. [Google Scholar] [CrossRef]
- Yanai, H.; Murakami, T.; Yoshiyama, H.; Takeuchi, H.; Nishikawa, J.; Nakamura, H.; Okita, K.; Miura, O.; Shimizu, N.; Takada, K. Epstein-Barr virus-associated gastric carcinoma and atrophic gastritis. J. Clin. Gastroenterol. 1999, 29, 39–43. [Google Scholar] [CrossRef]
- Iizasa, H.; Ishihara, S.; Ricardo, T.; Kanehiro, Y.; Yoshiyama, H. Dysbiotic infection in the stomach. World J. Gastroenterol. 2015, 21, 11450–11457. [Google Scholar] [CrossRef]
- Imai, S.; Koizumi, S.; Sugiura, M.; Tokunaga, M.; Uemura, Y.; Yamamoto, N.; Tanaka, S.; Sato, E.; Osato, T. Gastric carcinoma: Monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc. Natl. Acad. Sci. USA 1994, 91, 9131–9135. [Google Scholar] [CrossRef] [Green Version]
- Yanai, H.; Iizasa, H.; Chihara, D.; Murakami, T.; Nishikawa, J.; Yoshiyama, H. Epstein-Barr virus detection using gastric biopsy specimens after rapid urease test for Helicobacter pylori. Endosc. Int. Open 2019, 7, E431–E432. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Morita, M.; Yabuta, Y.; Kuzushima, K.; Kato, K.; Kojima, S.; Matsuyama, T.; Morishima, T. Quantitative analysis of Epstein-Barr virus load by using a real-time PCR assay. J. Clin. Microbiol. 1999, 29, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Snyder, A.R.; Lieberman, P.M. Epstein-Barr virus episome stability is coupled to a delay in replication timing. J. Virol. 2009, 85, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, A.; Yanai, H.; Shimizu, N.; Okamoto, T.; Matsubara, Y.; Yamamoto, K.; Okita, K. Evaluation of Epstein-Barr virus DNA load in gastric mucosa with chronic atrophic gastritis using a real-time quantitative PCR assay. Int. J. Gastrointest. Cancer 2003, 34, 87–94. [Google Scholar] [CrossRef]
- Maruo, S.; Yang, L.; Takada, K. Roles of Epstein-Barr virus glycoproteins gp350 and gp25 in the infection of human epithelial cells. J. Gen. Virol. 2001, 82, 2373–2383. [Google Scholar] [CrossRef]
- Kimura, K.; Takemoto, T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969, 3, 87–97. [Google Scholar] [CrossRef]
- Kanakry, J.A.; Li, H.; Gellert, L.L.; Lemas, M.V.; Hsieh, W.; Hong, F.; Tan, K.L.; Gascoyne, R.D.; Gordon, L.I.; Fisher, R.I.; et al. Plasma Epstein-Barr virus DNA predicts outcome in advanced Hodgkin lymphoma: Correlative analysis from a large North American cooperative group trial. Blood 2013, 121, 3547–3553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inazawa, N.; Hori, T.; Hatakeyama, N.; Yamamoto, M.; Yoto, Y.; Nojima, M.; Suzuki, N.; Shimizu, N.; Tsutsumi, H. Large-scale multiplex polymerase chain reaction assay for diagnosis of viral reactivations after allogeneic hematopoietic stem cell transplantation. J. Med. Virol. 2015, 87, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Chen, Q.; Gibbings, D.; Sah, D.W.; Bumcrot, D. Tissue-specific gene silencing monitored in circulating RNA. RNA 2014, 20, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abadi, A.T.B. Diagnosis of helicobacter pylori using invasive and noninvasive approaches. J. Pathog. 2018, 2018, 9064952. [Google Scholar]
- Foroutan, M.; Loloei, B.; Irvani, S.; Azargashb, E. Accuracy of rapid urease test in diagnosing Helicobacter pylori infection in patients using NSAIDs. Saudi J. Gastroenterol. 2010, 16, 110–112. [Google Scholar] [CrossRef]
- Abadi, A.T.B.; Taghvaei, T.; Wolfram, L. Inefficiency of rapid urease test for confirmation of Helicobacter pylori. Saudi J. Gastroenterol. 2011, 17, 84–85. [Google Scholar] [CrossRef] [PubMed]
- Minoura-Etoh, J.; Gotoh, K.; Sato, R.; Ogata, M.; Kaku, N.; Fujioka, T.; Nishizono, A. Helicobacter pylori-associated oxidant monochloramine induces reactivation of Epstein-Barr virus (EBV) in gastric epithelial cells latently infected with EBV. J. Med. Microbiol. 2006, 55, 905–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristic | Biopsy Samples (N = 58) |
|---|---|
| Gender | |
| Female (%) | 25 (43.1) |
| Male (%) | 33 (56.9) |
| Median age (years) (range) | 63 (26–93) |
| Atrophic gastritis grade (Kimura–Takemoto classification) | |
| C1 (mild) (%) | 13 (22.4) |
| C2 (mild) (%) | 14 (24.1) |
| C3 (moderate) (%) | 10 (17.2) |
| O1 (moderate) (%) | 15 (25.9) |
| O2 (severe) (%) | 3 (5.2) |
| O3 (severe) (%) | 3 (5.2) |
| H. pylori infection (RUT) | |
| H. pylori positive (%) | 44 (75.9) |
| H. pylori negative (%) | 14 (24.1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartika, A.V.; Iizasa, H.; Ding, D.; Kanehiro, Y.; Tajima, Y.; Kaji, S.; Yanai, H.; Yoshiyama, H. Application of Biopsy Samples Used for Helicobacter pylori Urease Test to Predict Epstein–Barr Virus-Associated Cancer. Microorganisms 2020, 8, 923. https://doi.org/10.3390/microorganisms8060923
Kartika AV, Iizasa H, Ding D, Kanehiro Y, Tajima Y, Kaji S, Yanai H, Yoshiyama H. Application of Biopsy Samples Used for Helicobacter pylori Urease Test to Predict Epstein–Barr Virus-Associated Cancer. Microorganisms. 2020; 8(6):923. https://doi.org/10.3390/microorganisms8060923
Chicago/Turabian StyleKartika, Andy Visi, Hisashi Iizasa, Dan Ding, Yuichi Kanehiro, Yoshitsugu Tajima, Shunsuke Kaji, Hideo Yanai, and Hironori Yoshiyama. 2020. "Application of Biopsy Samples Used for Helicobacter pylori Urease Test to Predict Epstein–Barr Virus-Associated Cancer" Microorganisms 8, no. 6: 923. https://doi.org/10.3390/microorganisms8060923