Molecular Identification of Borrelia afzelii from Ticks Parasitizing Domestic and Wild Animals in South Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Tick Collection and Species Identification
2.3. Molecular Detection of Ticks and TBPs
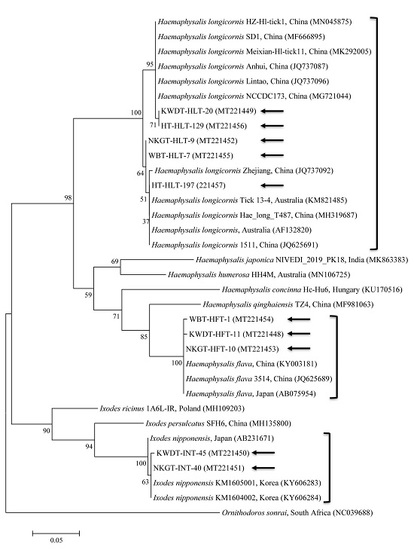
2.4. DNA Cloning, Nucleotide Sequencing, and Phylogenetic Analysis
3. Results
3.1. Identification of Ticks
3.2. Identification of Borrelia spp.
3.3. Molecular and Phylogenetic Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baneth, G. Tick-borne infections of animals and humans: A common ground. Int. J. Parasitol. 2014, 44, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.J.; Kim, H.; Won, S.; Kim, H.C.; Chong, S.T.; Klein, T.A.; Kim, K.G.; Seo, H.Y.; Chae, J.S. Ticks collected from wild and domestic animals and natural habitats in the Republic of Korea. Korean J. Parasitol. 2014, 52, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Aguero-Rosenfeld, M.E.; Wang, G.; Schwartz, I.; Wormser, G.P. Diagnosis of lyme borreliosis. Clin. Microbiol. Rev. 2005, 18, 484–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; van Dam, A.P.; Schwartz, I.; Dankert, J. Molecular typing of Borrelia burgdorferi sensu lato: Taxonomic, epidemiological, and clinical implications. Clin. Microbiol. Rev. 1999, 12, 633–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwan, T.G.; Raffel, S.J.; Schrumpf, M.E.; Schrumpf, M.E.; Webster, L.S.; Marques, A.R.; Spano, R.; Rood, M.; Burns, J.; Hu, R. Tick-borne relapsing fever and Borrelia hermsii, Los Angeles County, California, USA. Emerg. Infect. Dis. 2009, 15, 1026–1031. [Google Scholar] [CrossRef]
- Stanek, G.; Reiter, M. The expanding Lyme Borrelia complex--clinical significance of genomic species? Clin. Microbiol. Infect. 2011, 17, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Marconi, R.T.; Liveris, D.; Schwartz, I. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: Phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J. Clin. Microbiol. 1995, 33, 2427–2434. [Google Scholar]
- Kawabata, H.; Masuzawa, T.; Yanagihara, Y. Genomic analysis of Borrelia japonica sp. nov. isolated from Ixodes ovatus in Japan. Microbiol. Immunol. 1993, 37, 843–848. [Google Scholar] [CrossRef]
- Fukunaga, M.; Hamase, A.; Okada, K.; Nakao, M. Borrelia tanukii sp. nov. and Borrelia turdae sp. nov. found from ixodid ticks in Japan: Rapid species identification by 16S rRNA gene-targeted PCR analysis. Microbiol. Immunol. 1996, 40, 877–881. [Google Scholar] [CrossRef]
- Masuzawa, T.; Takada, N.; Kudeken, M.; Fukui, T.; Yano, Y.; Ishiguro, F.; Kawamura, Y.; Imai, Y.; Ezaki, T. Borrelia sinica sp. nov., a lyme disease-related Borrelia species isolated in China. Int. J. Syst. Evol. Microbiol. 2001, 51, 1817–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.J.; Han, S.H.; Park, J.M.; Lee, K.M.; Lee, E.M.; Lee, S.H.; Song, H.J.; Koh, Y.S.; Lee, K.W.; Jang, W.J.; et al. First molecular detection of Borrelia afzelii in clinical samples in Korea. Microbiol. Immunol. 2007, 51, 1201–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, J.S.; Yu, D.H.; Shringi, S.; Klein, T.A.; Kim, H.C.; Chong, S.T.; Lee, I.Y.; Foley, J. Microbial pathogens in ticks, rodents and a shrew in northern Gyeonggi-do near the DMZ, Korea. J. Vet. Sci. 2008, 9, 285–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.G.; Ko, S.; Smith, W.B.; Kim, H.C.; Lee, I.Y.; Chae, J.S. Prevalence of Anaplasma, Bartonella and Borrelia Species in Haemaphysalis longicornis collected from goats in North Korea. J. Vet. Sci. 2016, 17, 207–216. [Google Scholar] [CrossRef] [PubMed]
- VanBik, D.; Lee, S.H.; Seo, M.G.; Jeon, B.R.; Goo, Y.K.; Park, S.J.; Rhee, M.H.; Kwon, O.D.; Kim, T.H.; Geraldino, P.J.L.; et al. Borrelia species detected in ticks feeding on wild Korean water deer (Hydropotes inermis) using molecular and genotypic analyses. J. Med. Entomol. 2017, 54, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.M.; Seo, J.W.; Kim, D.M.; Yun, N.R.; Park, J.W.; Chung, J.K.; Song, H.J. Detection of Borrelia miyamotoi in Ixodes nipponensis in Korea. PLoS ONE 2019, 14, e0220465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, S.; Cha, R.M.; Yu, D.S.; Kim, K.S.; Song, S.; Choi, S.H.; Jung, B.I.; Lim, S.I.; Hyun, B.H.; Park, B.K.; et al. Rapid spread of classical swine fever virus among South Korean wild boars in areas near the border with North Korea. Pathogens 2020, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Agriculture, Food and Rural Affairs, South Korea (MAFRA). Report of “A Fact Finding Survey of Horse Industry in South Korea during 2018”; Ministry of Agriculture, Food and Rural Affairs: Sejong, Korea, 2019.
- Ministry of Agriculture, Food and Rural Affairs, South Korea (MAFRA). Major Statistics of Agriculture, Food and Rural Affairs during 2019; Ministry of Agriculture, Food and Rural Affairs: Sejong, Korea, 2019.
- Barker, S.C.; Walker, A.R. Ticks of Australia. The species that infest domestic animals and humans. Zootaxa 2014, 3816, 1–144. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Postic, D.; Assous, M.V.; Grimont, P.A.; Baranton, G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int. J. Syst. Bacteriol. 1994, 44, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Chae, J.B.; Cho, Y.S.; Cho, Y.K.; Kang, J.G.; Shin, N.S.; Chae, J.S. Epidemiological investigation of tick species from near domestic animal farms and cattle; goat; and wild boar in Korea. Korean. J. Parasitol. 2019, 57, 319–324. [Google Scholar]
- Seo, M.G.; Lee, S.H.; Ouh, I.O.; Lee, G.H.; Goo, Y.K.; Kim, S.; Kwon, O.D.; Kwak, D. molecular detection and genotyping of Coxiella-like endosymbionts in ticks that infest horses in South Korea. PLoS ONE 2016, 11, e0165784. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.T.; Kim, H.C.; Lee, I.Y.; Kollars, T.M., Jr.; Sancho, A.R.; Sames, W.J.; Chae, J.S.; Klein, T.A. Seasonal distribution of ticks in four habitats near the demilitarized zone, Gyeonggi-do (Province), Republic of Korea. Korean J. Parasitol. 2013, 51, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Baek, J.; Durey, A.; Kwon, H.Y.; Chung, M.H.; Lee, J.S. Current status of tick-borne diseases in South Korea. Vector Borne Zoonotic Dis. 2019, 19, 225–233. [Google Scholar] [CrossRef]
- Seong, G.; Han, Y.J.; Oh, S.S.; Chae, J.S.; Yu, D.H.; Park, J.; Park, B.K.; Yoo, J.G.; Choi, K.S. Detection of Tick-Borne Pathogens in the Korean Water Deer (Hydropotes inermis argyropus) from Jeonbuk Province, Korea. Korean J. Parasitol. 2015, 53, 653–659. [Google Scholar] [CrossRef]
- Seo, M.G.; Kwon, O.D.; Kwak, D. Molecular and phylogenetic analysis of tick-borne pathogens in ticks parasitizing native Korean goats (Capra hircus coreanae) in South Korea. Pathogens 2020, 9, 71. [Google Scholar] [CrossRef] [Green Version]
- Park, K.H.; Chang, W.H.; Schwan, T.G. Identification and characterization of Lyme disease spirochetes, Borrelia burgdorferi sensu lato, isolated in Korea. J. Clin. Microbiol. 1993, 31, 1831–1837. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.G.; Chung, K.Y.; Choi, Y.S.; Cho, S.N. Lyme disease. Korean J. Dermatol. 1993, 31, 601–605. [Google Scholar] [CrossRef] [Green Version]
- Kee, S.; Hwang, K.J.; Oh, H.B.; Kim, M.B.; Shim, J.C.; Ree, H.I.; Park, K.S. Isolation and identification of Borrelia burgdorferi in Korea. J. Korean Soc. Microbiol. 1994, 29, 301–310. [Google Scholar]
- Eisen, L. Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: A review. Ticks Tick Borne Dis. 2020, 11, 101359. [Google Scholar] [CrossRef]
- Breuner, N.E.; Ford, S.L.; Hojgaard, A.; Osikowicz, L.M.; Parise, C.M.; Rosales Rizzo, M.F.; Bai, Y.; Levin, M.L.; Eisen, R.J.; Eisen, L. Failure of the Asian longhorned tick, Haemaphysalis longicornis, to serve as an experimental vector of the Lyme disease spirochete, Borrelia burgdorferi sensu stricto. Ticks Tick Borne Dis. 2020, 11, 101311. [Google Scholar] [CrossRef] [PubMed]
- Korea Centers for Disease Control and Prevention (KCDC). Public health weekly report disease surveillance statistics. KCDC 2020, 13, 10. [Google Scholar]
- Pal, U.; Li, X.; Wang, T.; Montgomery, R.R.; Ramamoorthi, N.; Desilva, A.M.; Bao, F.; Yang, X.; Pypaert, M.; Pradhan, D.; et al. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 2004, 119, 457–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Divers, T.J.; Gardner, R.B.; Madigan, J.E.; Witonsky, S.G.; Bertone, J.J.; Swinebroad, E.L.; Schutzer, S.E.; Johnson, A.L. Borrelia burgdorferi Infection and Lyme Disease in North American Horses: A Consensus Statement. J. Vet. Intern. Med. 2018, 32, 617–632. [Google Scholar] [CrossRef] [Green Version]
- Laus, F.; Veronesi, F.; Passamonti, F.; Paggi, E.; Cerquetella, M.; Hyatt, D.; Tesei, B.; Fioretti, D.P. Prevalence of tick borne pathogens in horses from Italy. J. Vet. Med. Sci. 2013, 75, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Yun, S.H.; Choi, E.; Park, Y.S.; Lee, S.E.; Cho, G.J.; Kwon, O.D.; Kwak, D. Serological detection of Borrelia burgdorferi among horses in Korea. Korean J. Parasitol. 2016, 54, 97–101. [Google Scholar] [CrossRef]
- Meng, X.J.; Lindsay, D.S.; Sriranganathan, N. Wild boars as sources for infectious diseases in livestock and humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2697–2707. [Google Scholar] [CrossRef] [Green Version]
- Faria, A.S.; das Neves Paiva-Cardoso, M.; Nunes, M.; Carreira, T.; Vale-Gonçalves, H.M.; Veloso, O.; Coelho, C.; Cabral, J.A.; Vieira-Pinto, M.; Vieira, M.L. First Detection of Borrelia burgdorferi sensu lato DNA in Serum of the wild boar (Sus scrofa) in Northern Portugal by Nested-PCR. Ecohealth 2015, 12, 183–187. [Google Scholar] [CrossRef]
- Wodecka, B.; Rymaszewska, A.; Skotarczak, B. Host and pathogen DNA identification in blood meals of nymphal Ixodes ricinus ticks from forest parks and rural forests of Poland. Exp. Appl. Acarol. 2014, 62, 543–555. [Google Scholar] [CrossRef] [Green Version]
- Pacilly, F.C.; Benning, M.E.; Jacobs, F.; Leidekker, J.; Sprong, H.; Van Wieren, S.E.; Takken, W. Blood feeding on large grazers affects the transmission of Borrelia burgdorferi sensu lato by Ixodes ricinus. Ticks Tick Borne Dis. 2014, 5, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Furuno, K.; Lee, K.; Itoh, Y.; Suzuki, K.; Yonemitsu, K.; Kuwata, R.; Shimoda, H.; Watarai, M.; Maeda, K.; Takano, A. Epidemiological study of relapsing fever borreliae detected in Haemaphysalis ticks and wild animals in the western part of Japan. PLoS ONE 2017, 12, e0174727. [Google Scholar] [CrossRef] [PubMed]
- Adeolu, M.; Gupta, R.S. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: The emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Van Leeuwenhoek 2014, 105, 1049–1072. [Google Scholar] [PubMed]


| Species | Region | Stage | No. Tick Positive/Tick Pool (%) | ||||
|---|---|---|---|---|---|---|---|
| Horse | Wild Boar | Native Korean Goat | Korean Water Deer | Total | |||
| Haemaphysalis longicornis | Northern | Nymph | 0 | 0 | 0 | 0/10 | 0/10 |
| Adult | 0 | 0/2 | 0 | 0/24 | 0/26 | ||
| Central | Nymph | 0 | 0 | 0 | 0/11 | 0/11 | |
| Adult | 0 | 0/1 | 0 | 0/22 | 0/23 | ||
| Southern | Nymph | 0 | 0 | 0/12 | 0/13 | 0/25 | |
| Adult | 0 | 0/2 | 1/10 (10) | 0/28 | 1/40 (2.5) | ||
| Jeju Island | Nymph | 0 | 0 | 0 | 0 | 0 | |
| Adult | 2/147 (1.4) | 0 | 0 | 0 | 2/147 (1.4) | ||
| Subtotal | Nymph | 0 | 0 | 0/12 | 0/34 | 0/46 | |
| Adult | 2/147 (1.4) | 0/5 | 1/10 (10) | 0/74 | 3/236 (0.9) | ||
| Haemaphysalis flava | Central | Nymph | 0 | 0 | 0 | 0/1 | 0/1 |
| Adult | 0 | 0/4 | 0 | 0/4 | 0/8 | ||
| Southern | Nymph | 0 | 0 | 0/3 | 0/1 | 0/4 | |
| Adult | 0 | 1/10 (10) | 0/4 | 0/6 | 1/20 (5) | ||
| Subtotal | Nymph | 0 | 0 | 0/3 | 0/2 | 0/5 | |
| Adult | 0 | 1/14 (7.1) | 0/4 | 0/10 | 1/28 (3.6) | ||
| Ixodes nipponensis | Central | Nymph | 0 | 0 | 0 | 0/1 | 0/1 |
| Adult | 0 | 0 | 0 | 1/4 (25) | 1/4 (25) | ||
| Southern | Nymph | 0 | 0 | 0/3 | 0 | 0/3 | |
| Adult | 0 | 0 | 1/2 (50) | 0/4 | 1/6 (16.7) | ||
| Subtotal | Nymph | 0 | 0 | 0/3 | 0/1 | 0/4 | |
| Adult | 0 | 0 | 1/2 (50) | 1/8 (12.5) | 2/10 (20) | ||
| Total | 2/147 (1.4) | 1/19 (5.3) | 2/34 (5.9) | 1/129 (0.8) | 6/329 (1.8) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.-G.; Kwon, O.-D.; Kwak, D. Molecular Identification of Borrelia afzelii from Ticks Parasitizing Domestic and Wild Animals in South Korea. Microorganisms 2020, 8, 649. https://doi.org/10.3390/microorganisms8050649
Seo M-G, Kwon O-D, Kwak D. Molecular Identification of Borrelia afzelii from Ticks Parasitizing Domestic and Wild Animals in South Korea. Microorganisms. 2020; 8(5):649. https://doi.org/10.3390/microorganisms8050649
Chicago/Turabian StyleSeo, Min-Goo, Oh-Deog Kwon, and Dongmi Kwak. 2020. "Molecular Identification of Borrelia afzelii from Ticks Parasitizing Domestic and Wild Animals in South Korea" Microorganisms 8, no. 5: 649. https://doi.org/10.3390/microorganisms8050649