Biotechnological Production and Characterization of Extracellular Melanin by Streptomyces nashvillensis
Abstract
:1. Introduction
1.1. Melanins
1.2. Microbial Melanins
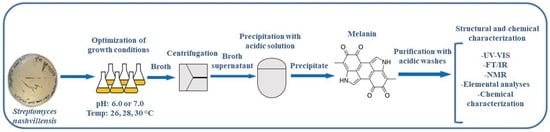
2. Materials and Methods
2.1. Materials
2.2. Microorganism and Media
2.3. Shake Flask Experiments
2.4. Melanin Purification
2.5. Tyrosinase Activity Assay
2.6. Melanin Quantification
2.7. Melanin Colorimetric Analysis
2.8. Melanin Structural Characterization
2.8.1. Melanin Elemental Analysis
2.8.2. Melanin Analysis by Fourier-Transform Infrared (FT-IR) Spectroscopy
2.8.3. Melanin Analysis by Nuclear Magnetic Resonance (NMR) Spectroscopy
2.9. Melanin Chemical Characterization
2.9.1. Melanin Solubility and Metal Chelating Activity
2.9.2. Melanin Thermal Stability and Resistance to UV-Visible Light Exposure
2.9.3. Redox Behavior and Antioxidant Activity
2.10. Data and Statistical Analysis
3. Results
3.1. Shake Flask Experiments
3.2. Melanin Purification and Structural Characterization
3.3. Melanin Chemical Characterization
4. Discussion
4.1. Melanin Production by Streptomycetes
4.2. Melanin Production by Streptomyces nashvillensis DSM 40314
5. Conclusions
Supplementary Materials
Author Contributions
Funding

Data Availability Statement
Conflicts of Interest
References
- Carletti, G.; Nervo, G.; Cattivelli, L. Flavonoids and melanins: A common strategy across two kingdoms. Int. J. Biol. Sci. 2014, 10, 1159–1170. [Google Scholar] [CrossRef]
- Madhusudhan, D.; Zainab Mazhari, B.B.; Dastager, S.G.; Agsar, D. Production and cytotoxicity of extracellular insoluble and droplets of soluble melanin by Streptomyces lusitans DMZ-3. BioMed Res. Int. 2014, 2014, 306895. [Google Scholar] [CrossRef]
- Solano, F. Melanin and melanin-related polymers as materials with biomedical and biotechnological applications-cuttlefish ink and mussel foot proteins as inspired biomolecules. Int. J. Mol. Sci. 2017, 18, 1561. [Google Scholar] [CrossRef]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin pigment in plants: Current knowledge and future perspectives. Front. Plant Sci. 2020, 11, 770. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Buehler, M. Melanin biopolymers: Tailoring chemical complexity for materials design. Angew. Chem. Int. Ed. 2020, 59, 11196–11205. [Google Scholar] [CrossRef]
- Al Khatib, M.; Harir, M.; Costa, J.; Baratto, M.C.; Schiavo, I.; Trabalzini, L.; Pollini, S.; Rossolini, G.M.; Basosi, R.; Pogni, R. Spectroscopic characterization of natural melanin from Streptomyces cyaneofuscatus strain and comparison with melanin enzymatically synthesized by tyrosinase and laccase. Molecules 2018, 23, 1916. [Google Scholar] [CrossRef]
- Mostert, A.B. Melanin, the what, the why and the how. Polymers 2021, 13, 1670. [Google Scholar] [CrossRef]
- Pezzella, A.; Barra, M.; Musto, A.; Navarra, A.; Alfè, M.; Manini, P.; Parisi, S.; Cassinese, A.; Criscuolo, V.; d’Ischia, M. Stem cell-compatible eumelanin biointerface fabricated by chemically controlled solid state polymerization. Mater. Horiz. 2015, 2, 212–220. [Google Scholar] [CrossRef]
- Manini, P.; Lucci, V.; Lino, V.; Sartini, S.; Rossella, F.; Falco, G.; Chiappe, C.; d’Ischia, M. Synthetic mycomelanin thin films as emergent bio-inspired interfaces controlling the fate of embryonic stem cells. J. Mater. Chem. B 2020, 8, 4412–4418. [Google Scholar] [CrossRef]
- Mbonyiryivuze, A.; Omollo, I.; Ngom, B.D.; Dhlamini, S.M.; Park, E.; Maaza, M. Natural dye sensitizer for grätzel cells: Sepia melanin. Phys. Mater. Chem. 2015, 3, 1–6. [Google Scholar]
- Sigma Aldrich. Available online: https://www.sigmaaldrich.com (accessed on 20 December 2023).
- Li, C.; Ji, C.; Tang, B. Purification, characterization, and biological activity of melanin from Streptomyces sp. FEMS Microbiol. Lett. 2018, 365, fny077. [Google Scholar] [CrossRef]
- Funa, N.; Funabashi, M.; Ohnishi, Y.; Horinouchi, S. Biosynthesis of hexahydroxyperylenequinone melanin via oxidative aryl coupling by cytochrome P-450 in Streptomyces griseus. J. Bacteriol. 2005, 187, 8149–8155. [Google Scholar] [CrossRef]
- Pralea, I.-E.; Moldovan, R.-C.; Petrache, A.-M.; Ilies, M.; Heghes, S.-C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Jang, S.; Sudheer, P.D.V.N.; Choi, K.-Y. Microbial production of melanin pigments from caffeic acid and L-tyrosine using Streptomyces glaucescens and FCS-ECH-expressing Escherichia coli. Int. J. Mol. Sci. 2021, 22, 2413. [Google Scholar] [CrossRef]
- Tran-Ly, A.N.; Reyes, C.; Schwarze, F.W.M.R.; Ribera, J. Microbial production of melanin and its various applications. World J. Microb. Biotechnol. 2020, 36, 170. [Google Scholar] [CrossRef]
- Barbuto Ferraiuolo, S.; Cammarota, M.; Schiraldi, C.; Restaino, O.F. Streptomycetes as platform for biotechnological production processes of drugs. Appl. Microbiol. Biotechnol. 2021, 105, 551–568. [Google Scholar] [CrossRef]
- Dholakiya, R.N.; Kumar, M.A.; Mody, K.H. Production and characterization of melanin from Streptomyces cavourensis strain RD8 using response surface optimization. Environ. Pollut. Prot. 2017, 2, 168–178. [Google Scholar] [CrossRef]
- El-Ahmady El-Naggar, N.; El-Ewasy, S.M. Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucenscens NEAE-H. Sci. Rep. 2017, 7, 42129. [Google Scholar] [CrossRef]
- Guo, J.; Rao, Z.; Yang, T.; Yang, T.; Man, Z.; Xu, M.; Zhang, X. High-level production of melanin by a novel isolate of Streptomyces kathirae. FEMS Microbiol. Lett. 2014, 357, 85–91. [Google Scholar] [CrossRef]
- Restaino, O.F.; Scognamiglio, M.; Mirpoor, S.F.; Cammarota, M.; Ventriglia, R.; Giosafatto, C.V.L.; Fiorentino, A.; Porta, R.; Schiraldi, C. Enhanced Streptomyces roseochromogenes melanin production by using the marine renewable source Posidonia oceanica egagropili. Appl. Microbiol. Biotechnol. 2022, 106, 7265–7283. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Li, Y. Metal ions driven production, characterization and bioactivity of extracellular melanin from Streptomyces sp. ZL-24. Int. J. Biol. Macromol. 2019, 123, 521–530. [Google Scholar] [CrossRef]
- Restaino, O.F.; Giosafatto, C.V.L.; Mirpoor, S.F.; Cammarota, M.; Hejazi, S.; Mariniello, L.; Schiraldi, C.; Porta, R. Sustainable exploitation of Posidonia oceanica sea balls (Egagropili): A Review. Int. J. Mol. Sci. 2023, 24, 7301. [Google Scholar] [CrossRef]
- Ikeda, Y.; Naganawa, H.; Kondo, S.; Takeuchi, T. Biosynthesis of bellenamine by Streptomyces nashvillensis using stable isotope labeled compounds. J. Antibiot. 1992, 45, 1919–1924. [Google Scholar] [CrossRef]
- Restaino, O.F.; Marseglia, M.; De Castro, C.; Diana, P.; Forni, P.; Parrilli, M.; De Rosa, M.; Schiraldi, C. Biotechnological transformation of hydrocortisone to 16α-hydroxy hydrocortisone by Streptomyces roseochromogenes. Appl. Microbiol. Biotechnol. 2014, 98, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Masterman, D.; Redding, K. Advanced Biology with Vernier: Experiments for AP and College General Biology; Vernier Software and Technology: Beaverton, OR, USA, 2010. [Google Scholar]
- Claus, H.; Decker, H. Bacterial tyrosinases. Syst. Appl. Microbiol. 2006, 29, 3–14. [Google Scholar] [CrossRef]
- Leu, W.M.; Chen, L.Y.; Liaw, L.L.; Lee, Y.H. Secretion of the Streptomyces tyrosinase is mediated through its trans-activator protein, MelC1. J. Biol. Chem. 1992, 267, 20108–20113. [Google Scholar] [CrossRef]
- Chen, L.Y.; Chen, M.Y.; Leu, W.M.; Tsai, T.Y.; Lee, Y.H. Mutational study of Streptomyces tyrosinase trans-activator MelC1. MelC1 is likely a chaperone for apotyrosinase. J. Biol. Chem. 1993, 268, 18710–18716. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chen, B.F.; Wu, S.Y.; Leu, W.M.; Lin, J.J.; Chen, C.W.; Lo, S.C. A trans-acting gene is required for the phenotypic expression of a tyrosinase gene in Streptomyces. Gene 1988, 65, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Liaw, L.L.; Lee, Y.H. Histidine residues 102 and 117 of MelC1 play different roles in the chaperone function for Streptomyces apotyrosinase. Biochem. Biophys. Res. Commun. 1995, 214, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Leu, W.M.; Wang, K.T.; Lee, Y.H. Copper transfer and activation of the Streptomyces apotyrosinase are mediated through a complex formation between apotyrosinase and its trans-activator MelC1. J. Biol. Chem. 1992, 267, 20100–20107. [Google Scholar] [CrossRef]
- Tseng, H.C.; Lin, C.K.; Hsu, B.J.; Leu, W.M.; Lee, Y.H.; Chiou, S.J.; Hu, N.T.; Chen, C.W. The melanin operon of Streptomyces antibioticus: Expression and use as a marker in gram-negative bacteria. Gene 1990, 86, 123–128. [Google Scholar] [CrossRef]
- Endo, K.; Kamo, K.; Hosono, K.; Beppu, T.; Ueda, K. Characterization of mutants defective in melanogenesis and a gene for tyrosinase of Streptomyces griseus. J. Antibiot. 2001, 54, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.P.; Lee, N.; Kim, B.G. Effect of Extracellular Tyrosinase on the Expression Level of P450, Fpr, and Fdx and Ortho-hydroxylation of Daidzein in Streptomyces avermitilis. Appl. Biochem. Biotechnol. 2017, 184, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Kim, E.J.; Kim, B.G. Regioselective Hydroxylation of trans-Resveratrol via Inhibition of Tyrosinase from Streptomyces avermitilis MA4680. ACS Chem. Biol. 2012, 7, 1687–1692. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Lee, Y.H. Roles of copper ligands in the activation and secretion of Streptomyces tyrosinase. J. Biol. Chem. 1998, 273, 19243–19250. [Google Scholar] [CrossRef]
- Wibowo, J.T.; Kellermann, M.Y.; Petersen, L.-E.; Alfiansah, Y.R.; Lattyak, C.; Schupp, P.J. Characterization of an insoluble and soluble form of melanin produced by Streptomyces cavourensis SV 21, a sea cucumber associated bacterium. Mar. Drugs 2022, 20, 54. [Google Scholar] [CrossRef] [PubMed]






| Melanin by S. nashvillensis | |
|---|---|
| Carbon (C, % w) | 60.1 |
| Hydrogen (H, % w) | 8.3 |
| Nitrogen (N, % w) | 9.8 |
| Others (O, % w) | 21.8 |
| Solubility | Solvent | Yes/No |
|---|---|---|
| H2O | Partly | |
| 1.0 M NaOH | Yes | |
| 1.0 M HCl | No | |
| Methanol | No | |
| Ethanol | No | |
| Acetone | No | |
| DMSO | Partly | |
| Chelating ability | Metal ion | Yes/no |
| 10.0 mM Na+ | No | |
| 10.0 mM K+ | No | |
| 10.0 mM Ca++ | No | |
| 10.0 mM Mg++ | No | |
| 10.0 mM Fe++ | Yes | |
| 10.0 mM Cu++ | Yes | |
| 10.0 mM Ni++ | Yes | |
| 10.0 mM Zn++ | Yes | |
| H2O2 (0.1–3.0%) oxidability | h | % |
| 48 h | From 1.56 ± 0.05 to 8.45 ± 0.03 | |
| Na2S2O3 (0.1–3.0%) reducibility | h | % |
| 48 h | From 0.41 ± 0.01 to 5.79 ± 0.01 | |
| Antioxidant activity | Melanin (g/L) | %η |
| 0.2 | 93.5 ± 0.1 | |
| 0.4 | 95.2 ± 0.1 | |
| 0.8 | 95.1 ± 0.1 | |
| 1.0 | 96.9 ± 0.1 | |
| Thermal stability | °C | % |
| 40 °C | 80.4 ± 0.1 | |
| 60 °C | 77.8 ± 0.1 | |
| 80 °C | 60.2 ± 0.1 | |
| 100 °C | 53.0 ± 0.1 | |
| h | % | |
| UV light resistance | 6 h | 83.4 ± 0.1 |
| VIS light resistance | 6 h | 80.4 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restaino, O.F.; Manini, P.; Kordjazi, T.; Alfieri, M.L.; Rippa, M.; Mariniello, L.; Porta, R. Biotechnological Production and Characterization of Extracellular Melanin by Streptomyces nashvillensis. Microorganisms 2024, 12, 297. https://doi.org/10.3390/microorganisms12020297
Restaino OF, Manini P, Kordjazi T, Alfieri ML, Rippa M, Mariniello L, Porta R. Biotechnological Production and Characterization of Extracellular Melanin by Streptomyces nashvillensis. Microorganisms. 2024; 12(2):297. https://doi.org/10.3390/microorganisms12020297
Chicago/Turabian StyleRestaino, Odile Francesca, Paola Manini, Talayeh Kordjazi, Maria Laura Alfieri, Massimo Rippa, Loredana Mariniello, and Raffaele Porta. 2024. "Biotechnological Production and Characterization of Extracellular Melanin by Streptomyces nashvillensis" Microorganisms 12, no. 2: 297. https://doi.org/10.3390/microorganisms12020297