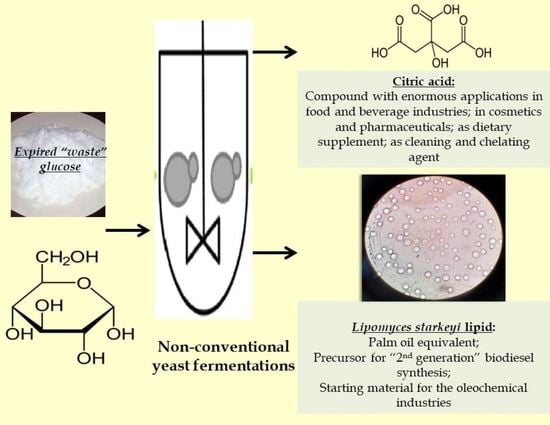
Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 1: High Production of Lipid by Lipomyces starkeyi and Citric Acid by Yarrowia lipolytica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Media
2.2. Culture Conditions
2.3. Analytical Methods
2.4. Data Analysis
3. Results and Discussion
3.1. Initial Screening of Yeast Strains on Glucose Base Media
3.2. Culture of L. starkeyi at Higher Initial Glucose Media
3.3. Citric Acid Production by Y. lipolytica in Bioreactor Experiments
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Papanikolaou, S.; Aggelis, G. Microbial products from wastes and residues. FEMS Microbiol. Lett. 2020, 367, fnaa156. [Google Scholar] [CrossRef]
- Valdés, G.; Mendonça, R.T.; Aggelis, G. Lignocellulosic biomass as a substrate for oleaginous microorganisms: A review. Appl. Sci. 2020, 10, 7698. [Google Scholar] [CrossRef]
- Pilafidis, S.; Diamantopoulou, P.; Gkatzionis, K.; Sarris, D. Valorization of agro-industrial wastes and residues through the production of bioactive compounds by macrofungi in liquid state cultures: Growing circular economy. Appl. Sci. 2022, 12, 11426. [Google Scholar] [CrossRef]
- Lin, C.S.; Koutinas, A.A.; Stamatelatou, K.; Mubofu, E.B.; Matharu, A.S.; Kopsahelis, N.; Pfaltzgraff, L.A.; Clark, J.H.; Papanikolaou, S.; Kwan, T.H.; et al. Current and future trends in food waste valorization for the production of chemicals. materials and fuels: A global perspective. Biofuels Bioprod. Bioref. 2014, 8, 686–715. [Google Scholar] [CrossRef]
- Coma, M.; Chatzifragkou, A. Chemicals from food supply chain by-products and waste streams. Molecules 2019, 24, 978. [Google Scholar] [CrossRef] [Green Version]
- Abeln, F.; Chuck, C.J. The history, state of the art and future prospects for oleaginous yeast research. Microb. Cell Fact. 2021, 20, 221. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Muniglia, L.; Chevalot, I.; Aggelis, G.; Marc, I. Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J. Appl. Microbiol. 2002, 92, 737–744. [Google Scholar] [CrossRef]
- Cavallo, E.; Charreau, H.; Cerrutti, P.; Foresti, M.L. Yarrowia lipolytica: A model yeast for citric acid production. FEMS Yeast Res. 2017, 17, fox084. [Google Scholar] [CrossRef] [Green Version]
- McNeil, B.A.; Stuart, D.T. Lipomyces starkeyi: An emerging cell factory for production of lipids, oleochemicals and biotechnology applications. World J. Microbiol. Biotechnol. 2018, 34, 147. [Google Scholar] [CrossRef]
- Arbter, P.; Sinha, A.; Troesch, J.; Utesch, T.; Zeng, A.-P. Redox governed electro-fermentation improves lipid production by the oleaginous yeast Rhodosporidium toruloides. Bioresour. Technol. 2019, 294, 122122. [Google Scholar] [CrossRef]
- Filippousi, R.; Tsouko, E.; Mordini, K.; Ladakis, D.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Sustainable arabitol production by a newly isolated Debaryomyces prosopidis strain cultivated on biodiesel-derived glycerol. Carbon Resour. Convers. 2022, 5, 92–99. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Filippousi, R.; Antoniou, D.; Varfi, E.; Xenopoulos, E.; Sarris, D.; Papanikolaou, S. Production of added-value microbial metabolites during growth of yeast strains on media composed of biodiesel-derived crude glycerol and glycerol/xylose blends. FEMS Microbiol. Lett. 2020, 367, fnaa063. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Yanniotis, S.; Kookos, I.K.; Koutinas, A.A. Utilisation of by-products from sunflower-based biodiesel production processes for the production of fermentation feedstock. Waste Biomass Valor. 2013, 4, 529–537. [Google Scholar] [CrossRef]
- Sarantou, S.; Stoforos, N.G.; Kalantzi, O.; Papanikolaou, S. Biotechnological valorization of biodiesel-derived glycerol: Trials with the non-conventional yeasts Yarrowia lipolytica and Rhodosporidium sp. Carbon Resour. Convers. 2021, 4, 61–75. [Google Scholar] [CrossRef]
- Fakas, S.; Papanikolaou, S.; Galiotou-Panayotou, M.; Komaitis, M.; Aggelis, G. Lipids of Cunninghamella echinulata with emphasis to γ-linolenic acid distribution among lipid classes. Appl. Microbiol. Biotechnol. 2006, 73, 676–683. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Rontou, M.; Belka, A.; Athenaki, M.; Gardeli, C.; Mallouchos, A.; Kalantzi, O.; Koutinas, A.A.; Kookos, I.K.; Zeng, A.-P.; et al. Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng. Life Sci. 2017, 17, 262–281. [Google Scholar] [CrossRef]
- Argyropoulos, D.; Psallida, C.; Sitareniou, P.; Flemetakis, E.; Diamantopoulou, P. Biochemical evaluation of Agaricus and Pleurotus strains in batch cultures for production optimization of valuable metabolites. Microorganisms 2022, 10, 964. [Google Scholar] [CrossRef]
- Makri, A.; Fakas, S.; Aggelis, G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010, 101, 2351–2358. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Kampisopoulou, E.; Blanchard, F.; Rondags, E.; Gardeli, C.; Koutinas, A.A.; Chevalot, I.; Aggelis, G. Production of secondary metabolites through glycerol fermentation under carbon-excess conditions by the yeasts Yarrowia lipolytica and Rhodosporidium toruloides. Eur. J. Lipid Sci. Technol. 2017, 119, 1600507. [Google Scholar] [CrossRef]
- Holdsworth, J.E.; Veenhuis, M.; Ratledge, C. Enzyme activities in oleaginous yeasts accumulating and utilizing exogenous or endogenous lipids. Microbiology 1988, 134, 2907–2915. [Google Scholar] [CrossRef] [Green Version]
- Xenopoulos, E.; Giannikakis, I.; Chatzifragkou, A.; Koutinas, A.A.; Papanikolaou, S. Lipid production by yeasts growing on commercial xylose in submerged cultures with process water being partially replaced by olive mill wastewaters. Processes 2020, 8, 819. [Google Scholar] [CrossRef]
- Aggelis, G.; Sourdis, J. Prediction of lipid accumulation-degradation in oleaginous micro-organisms growing on vegetable oils. Antonie Leeuwenhoek 1997, 72, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Chatzifragkou, A.; Makri, A.; Belka, A.; Bellou, S.; Mavrou, M.; Mastoridou, M.; Mystrioti, P.; Onjaro, G.; Aggelis, G.; Papanikolaou, S. Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 2011, 36, 1097–1108. [Google Scholar] [CrossRef]
- Sarris, D.; Galiotou-Panayotou, M.; Koutinas, A.A.; Komaitis, M.; Papanikolaou, S. Citric acid, biomass and cellular lipid production by Yarrowia lipolytica strains cultivated on olive mill wastewater-based media. J. Chem. Technol. Biotechnol. 2011, 86, 1439–1448. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Chatzifragkou, A.; Fakas, S.; Galiotou-Panayotou, M.; Komaitis, M.; Nicaud, J.-M.; Aggelis, G. Biosynthesis of lipids and organic acids by Yarrowia lipolytica strains cultivated on glucose. Eur. J. Lipid Sci. Technol. 2009, 111, 1221–1232. [Google Scholar] [CrossRef]
- Rymowicz, W.; Rywińska, A.; Żarowska, B.; Juszczyk, P. Citric acid production from raw glycerol by acetate mutants of Yarrowia lipolytica. Chem. Pap. 2006, 60, 391–394. [Google Scholar] [CrossRef]
- Rymowicz, W.; Rywińska, A.; Gładkowski, W. Simultaneous production of citric acid and erythritol from crude glycerol by Yarrowia lipolytica Wratislavia K1. Chem. Pap. 2008, 62, 239–246. [Google Scholar] [CrossRef]
- Rymowicz, W.; Fatykhova, A.R.; Kamzolova, S.V.; Rywińska, A.; Morgunov, I.G. Citric acid production from glycerol-containing waste of biodiesel industry by Yarrowia lipolytica in batch, repeated batch. and cell recycle regimes. Appl. Microbiol. Biotechnol. 2010, 87, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Stoforos, N.G.; Mallouchos, A.; Kookos, I.K.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Production of added-value metabolites by Yarrowia lipolytica growing in olive mill wastewater-based media under aseptic and non-aseptic conditions. Eng. Life Sci. 2017, 17, 695–709. [Google Scholar] [CrossRef] [Green Version]
- Bellou, S.; Triantaphyllidou, I.E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial oils as food additives: Recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Whiffin, F.; Santomauro, F.; Chuck, C.J. Toward a microbial palm oil substitute: Oleaginous yeasts cultured on lignocellulose: Towards a microbial palm oil substitute: Oleaginous yeasts cultured on lignocellulose. Biofuels Bioprod. Bioref. 2016, 10, 316–334. [Google Scholar] [CrossRef]
- Karayannis, D.; Papanikolaou, S.; Vatistas, C.; Paris, C.; Chevalot, I. Yeast lipid produced through glycerol conversions and its use for enzymatic synthesis of amino acid-based biosurfactants. Int. J. Mol. Sci. 2022, 24, 714. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.T.; Ratledget, C. The physiological significance of citric acid in the control of metabolism in lipid-accumulating yeasts. Biotechnol. Genet. Eng. Rev. 1985, 3, 349–376. [Google Scholar] [CrossRef]
- Tchakouteu, S.S.; Kalantzi, O.; Gardeli, C.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Lipid production by yeasts growing on biodiesel-derived crude glycerol: Strain selection and impact of substrate concentration on the fermentation efficiency. J. Appl. Microbiol. 2015, 118, 911–927. [Google Scholar] [CrossRef] [PubMed]
- Vamvakaki, A.-N.; Kandarakis, I.; Kaminarides, S.; Komaitis, M.; Papanikolaou, S. Cheese whey as a renewable substrate for microbial lipid and biomass production by Zygomycetes. Eng. Life Sci. 2010, 10, 348–360. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Aggelis, G.; Philippoussis, A. Adaptation of Volvariella volvacea metabolism in high carbon to nitrogen ratio media. Food Chem. 2016, 196, 272–280. [Google Scholar] [CrossRef]
- Tsakona, S.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Formulation of fermentation media from flour-rich waste streams for microbial lipid production by Lipomyces starkeyi. J. Biotechnol. 2014, 189, 36–45. [Google Scholar] [CrossRef]
- Giannakis, N.; Carmona-Cabello, M.; Makri, A.; Leiva-Candia, D.E.; Filippi, K.; Argeiti, C.; Pateraki, C.; Dorado, M.P.; Koutinas, A.A.; Stylianou, E. Spent coffee grounds and orange peel residues based biorefinery for microbial oil and biodiesel conversion estimation. Renew. Energy 2023, 209, 382–392. [Google Scholar] [CrossRef]
- Angerbauer, C.; Siebenhofer, M.; Mittelbach, M.; Guebitz, G.M. Conversion of sewage sludge into lipids by Lipomyces starkeyi for biodiesel production. Bioresour. Technol. 2008, 99, 3051–3056. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, X.; Hua, Y.; Feng, B.; Zhao, Z.K. Medium optimization for lipid production through co-fermentation of glucose and xylose by the oleaginous yeast Lipomyces starkeyi. Eur. J. Lipid Sci. Technol. 2008, 110, 405–412. [Google Scholar] [CrossRef]
- Ali El-Naggar, N.E.-A.; El-Hersh, M.S.; El-Fadaly, H.A.; Saber, W.I.A. Bioconversion of some agro-industrial by-products into single cell oil using Candida albicans NRRL Y-12983 and Lipomyces starkeyi NRRL Y-11557. Res. J. Microbiol. 2011, 6, 784–795. [Google Scholar]
- Gong, Z.; Wang, Q.; Shen, H.; Hu, C.; Jin, G.; Zhao, Z.K. Co-fermentation of cellobiose and xylose by Lipomyces starkeyi for lipid production. Bioresour. Technol. 2012, 117, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-X.; Yue, Q.-Y.; Gao, B.-Y.; Ma, Z.-H.; Zhang, P.-D. Microbial treatment of the monosodium glutamate wastewater by Lipomyces starkeyi to produce microbial lipid. Bioresour. Technol. 2012, 106, 69–73. [Google Scholar] [CrossRef]
- Matsakas, L.; Sterioti, A.A.; Rova, U.; Christakopoulos, P. Use of dried sweet sorghum for the efficient production of lipids by the yeast Lipomyces starkeyi CBS 1807. Ind. Crops Prod. 2014, 62, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Vieira, J.P.F.; Ienczak, J.L.; Rossell, C.E.V.; Pradella, J.G.C.; Franco, T.T. Microbial lipid production: Screening with yeasts grown on Brazilian molasses. Biotechnol. Lett. 2014, 36, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Anschau, A.; Xavier, M.C.A.; Hernalsteens, S.; Franco, T.T. Effect of feeding strategies on lipid production by Lipomyces starkeyi. Bioresour. Technol. 2014, 157, 214–222. [Google Scholar] [CrossRef]
- Salunke, D.; Manglekar, R.; Gadre, R.; Nene, S.; Harsulkar, A.M. Production of polyunsaturated fatty acids in recombinant Lipomyces starkeyi through submerged fermentation. Bioproc. Biosyst. Eng. 2015, 38, 1407–1414. [Google Scholar] [CrossRef]
- Probst, K.V.; Vadlani, P.V. Production of single cell oil from Lipomyces starkeyi ATCC 56304 using biorefinery by-products. Bioresour. Technol. 2015, 198, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Calvey, C.H.; Su, Y.-K.; Willis, L.B.; McGee, M.; Jeffries, T.W. Nitrogen limitation, oxygen limitation, and lipid accumulation in Lipomyces starkeyi. Bioresour. Technol. 2016, 200, 780–788. [Google Scholar] [CrossRef]
- Probst, K.V.; Vadlani, P.V. Single cell oil production by Lipomyces starkeyi: Biphasic fed-batch fermentation strategy providing glucose for growth and xylose for oil production. Biochem. Eng. J. 2017, 121, 49–58. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Fakas, S.; Fick, M.; Chevalot, I.; Galiotou-Panayotou, M.; Komaitis, M.; Marc, I.; Aggelis, G. Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: Production of 1,3-propanediol, citric acid and single cell oil. Biomass Bioenergy 2008, 32, 60–71. [Google Scholar] [CrossRef]
- Rywińska, A.; Rymowicz, W.; Żarowska, B.; Skrzypiński, A. Comparison of citric acid production from glycerol and glucose by different strains of Yarrowia lipolytica. World J. Microbiol. Biotechnol. 2010, 26, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Morgunov, I.G.; Aurich, A.; Perevoznikova, O.A.; Shishkanova, N.V.; Stottmeister, U.; Finogenova, T.V. Lipase secretion and citric acid production in Yarrowia lipolytica yeast grown on animal and vegetable fat. Food Technol. Biotechnol. 2005, 43, 113–122. [Google Scholar]
- Moeller, L.; Grünberg, M.; Zehnsdorf, A.; Aurich, A.; Bley, T.; Strehlitz, B. Repeated fed-batch fermentation using biosensor online control for citric acid production by Yarrowia lipolytica. J. Biotechnol. 2011, 153, 133–137. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. The citric acid production from raw glycerol by Yarrowia lipolytica yeast and its regulation. Appl. Microbiol. Biotechnol. 2013, 97, 7387–7397. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Diamantopoulou, P.; Blanchard, F.; Lambrinea, E.; Chevalot, I.; Stoforos, N.G.; Rondags, E. Physiological characterization of a novel wild-type Yarrowia lipolytica strain grown on glycerol: Effects of cultivation conditions and mode on polyols and citric acid production. Appl. Sci. 2020, 10, 7373. [Google Scholar] [CrossRef]
- Klasson, T.K.; Clausen, E.C.; Gaddy, J.L. Continuous fermentation for the production of citric acid from glucose. Appl. Biochem. Biotechnol. 1989, 20, 491–509. [Google Scholar] [CrossRef]
- Rane, K.D.; Sims, K.A. Production of citric acid by Candida lipolytica Y1095: Effect of glucose concentration on yield and productivity. Enzyme Microb. Technol. 1993, 15, 646–651. [Google Scholar] [CrossRef]
- Moresi, M. Effect of glucose concentration on citric acid production by Yarrowia lipolytica. J. Chem. Technol. Biotechnol. 1994, 60, 387–395. [Google Scholar] [CrossRef]
- Moeller, L.; Grünberg, M.; Zehnsdorf, A.; Strehlitz, B.; Bley, T. Biosensor online control of citric acid production from glucose by Yarrowia lipolytica using semicontinuous fermentation. Eng. Life Sci. 2010, 10, 311–320. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G. Metabolic peculiarities of the citric acid overproduction from glucose in yeasts Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.; Shishkanova, N.; Morgunov, I.; Finogenova, T. Oxygen requirements for growth and citric acid production of Yarrowia lipolytica. FEMS Yeast Res. 2003, 3, 217–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förster, A.; Aurich, A.; Mauersberger, S.; Barth, G. Citric acid production from sucrose using a recombinant strain of the yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2007, 75, 1409–1417. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Beopoulos, A.; Koletti, A.; Thévenieau, F.; Koutinas, A.A.; Nicaud, J.; Aggelis, G. Importance of the methyl-citrate cycle on glycerol metabolism in the yeast Yarrowia lipolytica. J. Biotechnol. 2013, 168, 303–314. [Google Scholar] [CrossRef]
- Morgunov, I.; Kamzolova, S.; Lunina, J. Citric acid production by Yarrowia lipolytica yeast on different renewable raw materials. Fermentation 2018, 4, 36. [Google Scholar] [CrossRef] [Green Version]




| Strain | Point | Time (h) | Glccons (g/L) | X (g/L) | L (g/L) | Lipid in DCW (%, w/w) |
|---|---|---|---|---|---|---|
| R. toruloides DSM 4444 | a, b | 168 | 48.8 ± 2.1 | 8.9 ± 0.9 | 4.9 ± 0.5 | 55.1 |
| R. glutinis NRRL YB-252 | a, b | 192 | 45.0 ± 2.2 | 11.9 ± 1.0 | 3.0 ± 0.4 | 25.3 |
| R. toruloides NRRL Y-27012 | a, b | 168 | 40.0 ± 3.6 | 11.4 ± 1.8 | 4.5 ± 0.7 | 39.5 |
| L. starkeyi DSM 70296 | a | 72 | 33.9 ± 2.2 | 13.8 ± 1.9 | 4.0 ± 0.6 | 28.9 |
| b | 168 | 44.1 ± 2.9 | 18.0 ± 2.0 | 2.9 ± 0.9 | 16.1 | |
| Y. lipolytica LFMB Y-20 | a | 24 | 7.1 ± 1.7 | 4.1 ± 0.9 | 0.7 ± 0.2 | 17.1 |
| b | 195 | 48.1 ± 2.1 | 7.4 ± 1.8 | 0.6 ± 0.2 | 8.1 | |
| Y. lipolytica ACA-DC 50109 | a | 26 | 8.2 ± 1.5 | 4.4 ± 0.8 | 0.8 ± 0.3 | 18.2 |
| b | 212 | 48.8 ± 0.9 | 6.4 ± 1.3 | 0.6 ± 0.3 | 9.4 |
| Yeast strain | C16:0 | C18:0 | Δ9C18:1 | Δ9,12C18:2 |
|---|---|---|---|---|
| R. toruloides DSM 4444 | 22.5 ± 2.1 | 8.5 ± 2.5 | 57.1 ± 4.1 | 7.4 ± 1.9 |
| R. glutinis NRRL YB-252 | 18.8 ± 2.8 | 2.1 ± 0.6 | 59.9 ± 5.5 | 2.1 ± 0.4 |
| R. toruloides NRRL Y-27012 | 30.4 ± 3.9 | 12.9 ± 2.1 | 50.7 ± 4.1 | 4.9 ± 0.9 |
| L. starkeyi DSM 70296 | 36.6 ± 3.6 | 5.4 ± 1.1 | 50.1 ± 5.1 | 1.6 ± 0.4 |
| Y. lipolytica LFMB Y-20 | 16.1 ± 1.9 | 7.3 ± 0.6 | 44.5 ± 2.6 | 18.7 ± 2.4 |
| Y. lipolytica ACA-DC 50109 | 14.9 ± 1.9 | 8.8 ± 1.6 | 46.9 ± 4.4 | 19.9 ± 2.6 |
| Quantity (%, w/w) | C16:0 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | |
|---|---|---|---|---|---|
| Total lipids (C/M 2/1 v/v) | 34.5 ± 4.1 | 7.1 ± 1.1 | 52.4 ± 5.2 | 1.8 ± 1.1 | |
| NLs | 89.6 ± 2.1 | 42.1 ± 4.9 | 8.1 ± 1.8 | 47.9 ± 4.4 | 1.6 ± 0.8 |
| G + S | 5.5 ± 1.9 | 39.8 ± 2.8 | 7.7 ± 1.9 | 48.1 ± 5.5 | 1.7 ± 0.5 |
| PLs | 4.9 ± 1.6 | 26.8 ± 2.5 | 4.4 ± 1.8 | 58.9 ± 3.1 | 3.0 ± 1.1 |
| Fermentation Time (h) | C16:0 | C18:0 | Δ9C18:1 | Δ9,12C18:2 |
|---|---|---|---|---|
| 48 | 34.1 ± 3.1 | 6.5 ± 2.1 | 47.1 ± 4.1 | 2.4 ± 0.9 |
| 72 | 37.2 ± 2.5 | 7.9 ± 3.0 | 49.9 ± 3.5 | 2.1 ± 0.4 |
| 135 | 40.9 ± 2.7 | 5.5 ± 2.1 | 51.7 ± 4.1 | 1.9 ± 0.5 |
| 192 | 38.1 ± 3.9 | 6.6 ± 2.8 | 52.1 ± 5.3 | 1.6 ± 0.4 |
| Strain | Culture Type | Substrate | X (g/L) | Lipid in DCW (%, w/w) | Reference |
|---|---|---|---|---|---|
| DSM 70295 | Batch shake flasks | Sewage sludge/glucose | 9.4 | 68.0 | Angerbauer et al. [40] |
| AS 2.1560 | Batch shake flasks | Glucose/xylose blend | 20.5 | 61.5 | Zhao et al. [41] |
| NRRL Y-11557 | Batch shake flasks | Molasses | 8.4 | 14.6 | El-Naggar et al. [42] |
| AS 2.1560 | Batch shake flasks | Cellobiose/xylose blend | 25.5 | 52.0 | Gong et al. [43] |
| GIM2.142 | Batch shake flasks | Glucose/MSGWW * | 4.6 | 24.7 | Liu et al. [44] |
| CBS 1807 | Batch shake flasks | Sweet sorghum | 21.7 | 29.5 | Matsakas et al. [45] |
| DSM 70296 | Fed-batch bioreactor | Molasses | 21.3 | 32.0 | Vieira et al. [46] |
| DSM 70296 | Fed-batch bioreactor | Sugar-cane bagasse | 85.4 | 49.0 | Anschau et al. [47] |
| DSM 70296 | Fed-batch bioreactor | Flour-waste hydrolysate | 109.8 | 57.8 | Tsakona et al. [38] |
| 3440 # | Fed-batch shake flasks | Glucose | 18.0 | 40.5 | Salunke et al. [48] |
| DSM 70296 | Batch shake flasks | Crude glycerol | 34.4 | 35.9 | Tchakouteu et al. [35] |
| ATCC 56304 | Batch shake flasks | Corn bran hydrolysate | 23.5 | 33.3 | Probst and Vadlani [49] |
| NRRL Y-11557 | Batch shake flasks | Corn stover hydrolysate | 24.6 | 38.7 | Calvey et al. [50] |
| ATCC 56304 | Fed-batch bioreactor | Glucose | 81.6 | 41.8 | Probst and Vadlani [51] |
| DSM 70296 | Fed-batch bioreactor | SCGH ** | 87.4 | 46.0 | Giannakis et al. [39] |
| DSM 70296 | Batch shake flasks | Expired glucose | 34.0 | 34.1 | Present study |
| Strain | Culture Type | Substrate | CA (g/L) | Yield (g/g) | Reference |
|---|---|---|---|---|---|
| Wild-type strains | |||||
| NRRL Y-7576 | Batch bioreactor | Glucose | 51.5 | 0.71 | Klasson et al. [58] |
| Y1095 | Fed-batch bioreactor | Glucose | 13.6–78.5 | 0.79 | Rane and Sims [59] |
| ATCC 20346 | Fed-batch bioreactor | Glucose | 50–69 | 0.52 | Moresi [60] |
| H222 | Batch bioreactor | Glucose | 62.0 | 0.37 | Moeller et al. [61] |
| A-101 | Batch bioreactor | Crude glycerol | 66.8 | 0.43 | Rywińska et al. [53] |
| H222 | Fed-batch batch bioreactor | Glucose | 97.7 | 0.56 | Moeller et al. [55] |
| VKM Y 2373 | Fed-batch bioreactor | Glucose | 80–85 | 0.70–0.75 | Kamzolova and Morgunov [62] |
| ACA YC 5029 | Batch bioreactor | Crude glycerol | 39.0 | 0.42 | Papanikolaou et al. [19] |
| LMBF Y-46 | Fed-batch bioreactor | Pure glycerol | 101.3 # | 0.46 | Papanikolaou et al. [57] |
| Mutants or genetically modified strains | |||||
| N1 | Fed-batch bioreactor | Ethanol | 120.0 | 0.85 | Kamzolova et al. [63] |
| 187/1 | Fed-batch bioreactor | Rapeseed oil | 135.0 | 1.55 | Kamzolova et al. [54] |
| Wratislavia AWG7 | Batch bioreactor | Crude glycerol | 88.1 | 0.46 | Rymowicz et al. [26] |
| H222-S4(p67ICL1)T5 | Fed-batch bioreactor | Sucrose | 133.0 # | 0.78 | Förster et al. [64] |
| A-101-1.22 | Fed-batch bioreactor | Crude glycerol | 119.1 # | 0.64 | Rymowicz et al. [28] |
| NG40/UV7 | Fed-batch bioreactor | Pure glycerol | 115.0 | 0.64 | Morgunov et al. [56] |
| JMY 1203 | Shake flasks | Crude glycerol | 57.7 # | 0.91 | Papanikolaou et al. [65] |
| NG40/UV5 | Fed-batch bioreactor | Rapeseed oil | 140.0 | 1.50 | Morgunov et al. [66] |
| ACA-DC 50109 | Fed-batch bioreactor | Expired glucose | 82.0 | 0.50 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diamantopoulou, P.; Sarris, D.; Tchakouteu, S.S.; Xenopoulos, E.; Papanikolaou, S. Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 1: High Production of Lipid by Lipomyces starkeyi and Citric Acid by Yarrowia lipolytica. Microorganisms 2023, 11, 1863. https://doi.org/10.3390/microorganisms11071863
Diamantopoulou P, Sarris D, Tchakouteu SS, Xenopoulos E, Papanikolaou S. Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 1: High Production of Lipid by Lipomyces starkeyi and Citric Acid by Yarrowia lipolytica. Microorganisms. 2023; 11(7):1863. https://doi.org/10.3390/microorganisms11071863
Chicago/Turabian StyleDiamantopoulou, Panagiota, Dimitris Sarris, Sidoine Sadjeu Tchakouteu, Evangelos Xenopoulos, and Seraphim Papanikolaou. 2023. "Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 1: High Production of Lipid by Lipomyces starkeyi and Citric Acid by Yarrowia lipolytica" Microorganisms 11, no. 7: 1863. https://doi.org/10.3390/microorganisms11071863