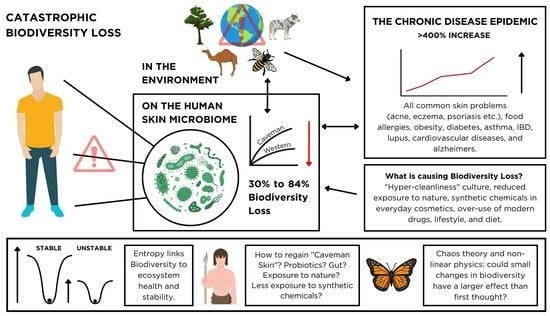
A Catastrophic Biodiversity Loss in the Environment Is Being Replicated on the Skin Microbiome: Is This a Major Contributor to the Chronic Disease Epidemic?
Abstract
:1. Introduction
2. Biodiversity Loss and the Skin Microbiome
2.1. Biodiversity Loss in Global Ecosystems
2.2. The Skin Microbiome, Biodiversity, and Dysbiosis
2.3. Are We Seeing a Loss of Biodiversity in the Human Skin Microbiome, Just like in the Environment and Our Gut?
2.4. What Is Causing the Biodiversity Loss in the Skin Microbiome?
3. Is Biodiversity Loss on the Skin Involved in the Chronic Disease Epidemic?
3.1. The Chronic Disease Epidemic
3.2. Skin Diseases Associated with Biodiversity Loss
3.3. Is Biodiversity Loss in the Skin Microbiome Associated with Systemic Diseases?
3.4. Biodiversity Loss: Cause or Symptom?
3.5. Potential Mechanisms of Chronic Disease Development
4. Biodiversity: The Link to Entropy and Ecosystem Health
- H = biodiversity index
- S = number of species encountered
- i = species
- pi = ni/N and describes “relative abundance”—the probability that a randomly chosen organism is of the ith species
- ni = total number of organisms of a particular species
- N = total number of organisms of all species
How Biodiversity Loss Negatively Affects Ecosystems
5. How Do We Regain the Lost Biodiversity?
6. Future Perspectives: Non-Linearity in Ecosystems and the Butterfly Effect
6.1. Could the Non-Linearity of Ecosystems Mean We Have Underestimated the Negative Effects of Biodiversity Loss?
6.2. Could Chaos Theory and the Butterfly Effect Mean We Have Underestimated the Interconnectedness of the Human Microbiome and Macro-Ecosystems?
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Jacobson, A.P.; Riggio, J.; Tait, A.M.; Baillie, E.M.J. Global Areas of Low Human Impact (‘Low Impact Areas’) and Fragmentation of the Natural World. Sci. Rep. 2019, 9, 14179. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D. Commentary on Chronic Disease Prevention in 2022. 2022. Available online: https://chronicdisease.org/wp-content/uploads/2022/04/FS_ChronicDiseaseCommentary2022FINAL.pdf (accessed on 30 September 2023).
- CDC. Health and Economic Costs of Chronic Diseases. Available online: https://www.cdc.gov/chronicdisease/about/costs/index.htm#print (accessed on 30 September 2023).
- WHO. Noncommunicable Diseases: Progress Monitor 2022. 2022. Available online: https://apps.who.int/iris/bitstream/handle/10665/353048/9789240047761-eng.pdf (accessed on 17 August 2023).
- Holman, H.R. The Relation of the Chronic Disease Epidemic to the Health Care Crisis. ACR Open Rheumatol. 2020, 2, 167. [Google Scholar] [CrossRef] [PubMed]
- Marrs, T.; Flohr, C. The Role of Skin and Gut Microbiota in the Development of Atopic Eczema. Br. J. Dermatol. 2016, 175, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Leclerc, M.; Hugot, J.P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of Antibiotics on the Human Microbiome and Consequences for Host Health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Methé, B.A.; Nelson, K.E.; Pop, M.; Creasy, H.H.; Giglio, M.G.; Huttenhower, C.; Gevers, D.; Petrosino, J.F.; Abubucker, S.; Badger, J.H.; et al. A Framework for Human Microbiome Research. Nature 2012, 486, 215. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The Skin Microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The Applied Anatomy of Human Skin: A Model for Regeneration. Wound Med. 2020, 28, 100179. [Google Scholar] [CrossRef]
- Querleux, B.; Rodrigues, L.M.; Jin, R.; Luo, L.; Zheng, J. The Trinity of Skin: Skin Homeostasis as a Neuro–Endocrine–Immune Organ. Life 2022, 12, 725. [Google Scholar] [CrossRef]
- Terui, H.; Yamasaki, K.; Wada-Irimada, M.; Onodera-Amagai, M.; Hatchome, N.; Mizuashi, M.; Yamashita, R.; Kawabe, T.; Ishii, N.; Abe, T.; et al. Staphylococcus aureus Skin Colonization Promotes SLE-like Autoimmune Inflammation via Neutrophil Activation and the IL-23/IL-17 Axis. Sci. Immunol. 2022, 7, eabm9811. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated Modern Human-Induced Species Losses: Entering the Sixth Mass Extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- Almond, R.E.A.; Grooten, M.; Petersen, T. Living Planet Report 2020—Bending the Curve of Biodiversity Loss; WWF: Gland, Switzerland, 2020. [Google Scholar]
- Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services. Popul. Dev. Rev. 2019, 45. [Google Scholar] [CrossRef]
- Davidson, N.C.; Davidson, N.C. How Much Wetland Has the World Lost? Long-Term and Recent Trends in Global Wetland Area. Mar. Freshw. Res. 2014, 65, 934–941. [Google Scholar] [CrossRef]
- Ellis, E.C.; Goldewijk, K.K.; Siebert, S.; Lightman, D.; Ramankutty, N. Anthropogenic Transformation of the Biomes, 1700 to 2000. Glob. Ecol. Biogeogr. 2010, 19, 589–606. [Google Scholar] [CrossRef]
- Plumptre, A.J.; Baisero, D.; Belote, R.T.; Vázquez-Domínguez, E.; Faurby, S.; Jȩdrzejewski, W.; Kiara, H.; Kühl, H.; Benítez-López, A.; Luna-Aranguré, C.; et al. Where Might We Find Ecologically Intact Communities? Front. For. Glob. Chang. 2021, 4, 26. [Google Scholar] [CrossRef]
- Souter, D.; Planes, S.; Wicquart, J.; Logan, M.; Obura, D.; Staub, F. Status of Coral Reefs of the World: 2020 Summary for Policymakers Summary for Policymakers-Status of Coral Reefs of the World: 2020 Value of Coral Reefs. 2020. Available online: https://gcrmn.net/wp-content/uploads/2022/05/Status-of-Coral-Reefs-of-the-World-2020-Summary-for-Policymakers.pdf (accessed on 12 November 2022).
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; et al. Impacts of Biodiversity Loss on Ocean Ecosystem Services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Guarner, F. The Gut Microbiota Era Marches On. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 647–649. [Google Scholar] [CrossRef]
- Ding, R.-X.; Goh, W.-R.; Wu, R.-N.; Yue, X.-Q.; Luo, X.; Khine, W.W.T.; Wu, J.-R.; Lee, Y.-K. Revisit Gut Microbiota and Its Impact on Human Health and Disease. J. Food Drug Anal. 2019, 27, 623–631. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, 36–44. [Google Scholar] [CrossRef]
- Brandwein, M.; Katz, I.; Katz, A.; Kohen, R. Beyond the Gut: Skin Microbiome Compositional Changes Are Associated with BMI. Hum. Microb. J. 2019, 13, 100063. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; Sivamani, R.K. The Skin and Gut Microbiome and Its Role in Common Dermatologic Conditions. Microorganisms 2019, 7, 550. [Google Scholar] [CrossRef] [PubMed]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut–Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Prescott, S.L.; Larcombe, D.-L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; van Etten, E.; Horwitz, P.; Kozyrskyj, A.; et al. The Skin Microbiome: Impact of Modern Environments on Skin Ecology, Barrier Integrity, and Systemic Immune Programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Čelakovská, J.; Bukač, J. The Severity of Atopic Dermatitis and Analysis of the Food Hypersensitivity Reactions. Food Agric. Immunol. 2015, 26, 896–908. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Fischbach, M.A. What Lives On Our Skin: Ecology, Genomics and Therapeutic Opportunities of the Skin Microbiome. Drug Discov. Today Dis. Mech. 2013, 10, e83–e89. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A. The Intersection of Microbiome and Host at the Skin Interface: Genomic- and Metagenomic-Based Insights. Genome Res. 2015, 25, 1514–1520. [Google Scholar] [CrossRef]
- Belkaid, Y.; Segre, J.A. Dialogue between Skin Microbiota and Immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial Community Variation in Human Body Habitats Across Space and Time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Byrd, A.L.; Deming, C.; Conlan, S.; Kong, H.H.; Segre, J.A.; Barnabas, B.; Blakesley, R.; Bouffard, G.; Brooks, S.; et al. Biogeography and Individuality Shape Function in the Human Skin Metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Degruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137. [Google Scholar] [CrossRef] [PubMed]
- Knights, D.; Lassen, K.G.; Xavier, R.J. Advances in Inflammatory Bowel Disease Pathogenesis: Linking Host Genetics and the Microbiome. Gut 2013, 62, 1505–1510. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Costello, E.K.; Berg-Lyons, D.; Gonzalez, A.; Stombaugh, J.; Knights, D.; Gajer, P.; Ravel, J.; Fierer, N.; et al. Moving Pictures of the Human Microbiome. Genome Biol. 2011, 12, R50. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Chng, K.R.; Tay, A.S.L.; Li, C.; Ng, A.H.Q.; Wang, J.; Suri, B.K.; Matta, S.A.; McGovern, N.; Janela, B.; Wong, X.F.C.C.; et al. Whole Metagenome Profiling Reveals Skin Microbiome-Dependent Susceptibility to Atopic Dermatitis Flare. Nat. Microbiol. 2016, 1, 16106. [Google Scholar] [CrossRef]
- Hsu, D.K.; Fung, M.A.; Chen, H.L. Role of Skin and Gut Microbiota in the Pathogenesis of Psoriasis, an Inflammatory Skin Disease. Med. Microecol. 2020, 4, 100016. [Google Scholar] [CrossRef]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Hvas, C.L.; Licht, T.R. Determining Gut Microbial Dysbiosis: A Review of Applied Indexes for Assessment of Intestinal Microbiota Imbalances. Appl. Environ. Microbiol. 2021, 87, e00395-21. [Google Scholar] [CrossRef] [PubMed]
- Lucini, F.A.; Morone, F.; Tomassone, M.S.; Makse, H.A. Diversity Increases the Stability of Ecosystems. PLoS ONE 2020, 15, e0228692. [Google Scholar] [CrossRef]
- Tilman, D.; Reich, P.B.; Knops, J.M.H. Biodiversity and Ecosystem Stability in a Decade-Long Grassland Experiment. Nature 2006, 441, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Yang, H.; Ros, V.; Roy, F.; Biroli, G.; Bunin, G.; Cammarota, C. Marginally Stable Equilibria in Critical Ecosystems. New J. Phys. 2018, 20, 083051. [Google Scholar] [CrossRef]
- Pasari, J.R.; Levi, T.; Zavaleta, E.S.; Tilman, D. Several Scales of Biodiversity Affect Ecosystem Multifunctionality. Proc. Natl. Acad. Sci. USA 2013, 110, 10219–10222. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut Microbiota: Role in Pathogen Colonization, Immune Responses and Inflammatory Disease. Immunol. Rev. 2017, 279, 70. [Google Scholar] [CrossRef]
- Srinivas, G.; Möller, S.; Wang, J.; Künzel, S.; Zillikens, D.; Baines, J.F.; Ibrahim, S.M. Genome-Wide Mapping of Gene–Microbiota Interactions in Susceptibility to Autoimmune Skin Blistering. Nat. Commun. 2013, 4, 2462. [Google Scholar] [CrossRef]
- Wallen-Russell, C.; Wallen-Russell, S. Meta Analysis of Skin Microbiome: New Link between Skin Microbiota Diversity and Skin Health with Proposal to Use This as a Future Mechanism to Determine Whether Cosmetic Products Damage the Skin. Cosmetics 2017, 4, 14. [Google Scholar] [CrossRef]
- Baldwin, H.E.; Bhatia, N.D.; Friedman, A.; Martin, R.; Seité, S. The Role of Cutaneous Microbiota Harmony in Maintaining a Functional Skin Barrier. J. Drugs Dermatol. 2017, 16, 12–18. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, S.; Barrett, M.; Kirthi, S.; Pellanda, P.; Vlckova, K.; Tobin, A.M.; Murphy, M.; Shanahan, F.; O’Toole, P.W. Altered Skin and Gut Microbiome in Hidradenitis Suppurativa. J. Investig. Dermatol. 2022, 142, 459–468.e15. [Google Scholar] [CrossRef]
- Carmona-Cruz, S.; Orozco-Covarrubias, L.; Sáez-de-Ocariz, M. The Human Skin Microbiome in Selected Cutaneous Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 834135. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tan, J.; Yang, H.; Gao, Z.; Cai, Q.; Meng, L.; Yang, L. Characterization of Skin Microbiome in Tinea Pedis. Indian J. Microbiol. 2019, 59, 422. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving Our Microbial Self: The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human Gut Microbiome Viewed across Age and Geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Lin, A.; Bik, E.M.; Costello, E.K.; Dethlefsen, L.; Haque, R.; Relman, D.A.; Singh, U. Distinct Distal Gut Microbiome Diversity and Composition in Healthy Children from Bangladesh and the United States. PLoS ONE 2013, 8, e53838. [Google Scholar] [CrossRef]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut Microbiome of the Hadza Hunter-Gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef]
- Carter, M.M.; Olm, M.R.; Merrill, B.D.; Dahan, D.; Tripathi, S.; Spencer, S.P.; Yu, F.B.; Jain, S.; Neff, N.; Jha, A.R.; et al. Ultra-Deep Sequencing of Hadza Hunter-Gatherers Recovers Vanishing Gut Microbes. Cell 2023, 186, 3111–3124.e13. [Google Scholar] [CrossRef]
- Sanders, J.G.; Sprockett, D.D.; Li, Y.; Mjungu, D.; Lonsdorf, E.V.; Ndjango, J.B.N.; Georgiev, A.V.; Hart, J.A.; Sanz, C.M.; Morgan, D.B.; et al. Widespread Extinctions of Co-Diversified Primate Gut Bacterial Symbionts from Humans. Nat. Microbiol. 2023, 8, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.; Stegen, J.C.; Maldonado-Gómez, M.X.; Eren, A.M.; Siba, P.M.; Greenhill, A.R.; Walter, J. The Gut Microbiota of Rural Papua New Guineans: Composition, Diversity Patterns, and Ecological Processes. Cell Rep. 2015, 11, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Pehrsson, E.C.; Blaser, M.J.; Sandhu, K.; Gao, Z.; Wang, B.; Magris, M.; Hidalgo, G.; Contreras, M.; Noya-Alarcón, Ó.; et al. The Microbiome of Uncontacted Amerindians. Sci. Adv. 2015, 1, e1500183. [Google Scholar] [CrossRef]
- Cordain, L.; Lindeberg, S.; Hurtado, M.; Hill, K.; Eaton, S.B.; Brand-Miller, J. Acne Vulgaris: A Disease of Western Civilization. Arch. Dermatol. 2002, 138, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Steiner, P.E. Necropsies on Okinawans. Anatomic and Pathologic Observations. Arch. Pathol. 1946, 42, 359–380. [Google Scholar]
- McCall, L.I.; Callewaert, C.; Zhu, Q.; Song, S.J.; Bouslimani, A.; Minich, J.J.; Ernst, M.; Ruiz-Calderon, J.F.; Cavallin, H.; Pereira, H.S.; et al. Home Chemical and Microbial Transitions across Urbanization. Nat. Microbiol. 2019, 5, 108–115. [Google Scholar] [CrossRef]
- Lehtimäki, J.; Karkman, A.; Laatikainen, T.; Paalanen, L.; Von Hertzen, L.; Haahtela, T.; Hanski, I.; Ruokolainen, L. Patterns in the Skin Microbiota Differ in Children and Teenagers between Rural and Urban Environments. Sci. Rep. 2017, 7, srep45651. [Google Scholar] [CrossRef]
- Blaser, M.J.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Estrada, I.; Gao, Z.; Clemente, J.C.; Costello, E.K.; Knight, R. Distinct Cutaneous Bacterial Assemblages in a Sampling of South American Amerindians and US Residents. ISME J. 2013, 7, 85–95. [Google Scholar] [CrossRef]
- Kennedy, E.A.; Connolly, J.; Hourihane, J.O.B.; Fallon, P.G.; McLean, W.H.I.; Murray, D.; Jo, J.H.; Segre, J.A.; Kong, H.H.; Irvine, A.D. Skin Microbiome before Development of Atopic Dermatitis: Early Colonization with Commensal Staphylococci at 2 Months Is Associated with a Lower Risk of Atopic Dermatitis at 1 Year. J. Allergy Clin. Immunol. 2017, 139, 166–172. [Google Scholar] [CrossRef]
- Misra, N.; Clavaud, C.; Guinot, F.; Bourokba, N.; Nouveau, S.; Mezzache, S.; Palazzi, P.; Appenzeller, B.M.R.; Tenenhaus, A.; Leung, M.H.Y.; et al. Multi-Omics Analysis to Decipher the Molecular Link between Chronic Exposure to Pollution and Human Skin Dysfunction. Sci. Rep. 2021, 11, 18302. [Google Scholar] [CrossRef]
- Wallen-Russell, C. The Role of Every-Day Cosmetics in Altering the Skin Microbiome: A Study Using Biodiversity. Cosmetics 2018, 6, 2. [Google Scholar] [CrossRef]
- Rocha, L.A.; Ferreira de Almeida e Borges, L.; Gontijo Filho, P.P. Changes in Hands Microbiota Associated with Skin Damage Because of Hand Hygiene Procedures on the Health Care Workers. Am. J. Infect. Control 2009, 37, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J.; Falkow, S. What Are the Consequences of the Disappearing Human Microbiota? Nat. Rev. Microbiol. 2009, 7, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Holland, K.T.; Bojar, R.A. Cosmetics: What Is Their Influence on the Skin Microflora? Am. J. Clin. Dermatol. 2002, 3, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin Microbiota: A Source of Disease or Defence? Br. J. Dermatol. 2009, 158, 442–455. [Google Scholar] [CrossRef]
- Stingley, R.L.; Zou, W.; Heinze, T.M.; Chen, H.; Cerniglia, C.E. Metabolism of Azo Dyes by Human Skin Microbiota. J. Med. Microbiol. 2010, 59, 108–114. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, T.; Pipal, A.; Redl, B. Molecular Analysis of the Prevalent Microbiota of Human Male and Female Forehead Skin Compared to Forearm Skin and the Influence of Make-Up. J. Appl. Microbiol. 2011, 110, 1381–1389. [Google Scholar] [CrossRef]
- Callewaert, C.; Knödlseder, N.; Karoglan, A.; Güell, M.; Paetzold, B. Skin Microbiome Transplantation and Manipulation: Current State of the Art. Comput. Struct. Biotechnol. J. 2021, 19, 624–631. [Google Scholar] [CrossRef]
- SanMiguel, A.J.; Meisel, J.S.; Horwinski, J.; Zheng, Q.; Grice, E.A. Topical Antimicrobial Treatments Can Elicit Shifts to Resident Skin Bacterial Communities and Reduce Colonization by Staphylococcus Aureus Competitors. Antimicrob. Agents Chemother. 2017, 61, e00774-17. [Google Scholar] [CrossRef]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am. J. Clin. Dermatol. 2020, 21, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J. Antibiotic Use and Its Consequences for the Normal Microbiome. Science 2016, 352, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Dorninger, C.; Abson, D.J.; Fischer, J.; von Wehrden, H. Assessing Sustainable Biophysical Human–Nature Connectedness at Regional Scales. Environ. Res. Lett. 2017, 12, 055001. [Google Scholar] [CrossRef]
- Birzele, L.T.; Depner, M.; Ege, M.J.; Engel, M.; Kublik, S.; Bernau, C.; Loss, G.J.; Genuneit, J.; Horak, E.; Schloter, M.; et al. Environmental and Mucosal Microbiota and Their Role in Childhood Asthma. Allergy 2017, 72, 109–119. [Google Scholar] [CrossRef]
- Amre, D.K.; Lambrette, P.; Law, L.; Krupoves, A.; Chotard, V.; Costea, F.; Grimard, G.; Israel, D.; Mack, D.; Seidman, E. Investigating the Hygiene Hypothesis as a Risk Factor in Pediatric Onset Crohn’s Disease. Am. J. Gastroenterol. 2006, 101, 1005–1111. [Google Scholar] [CrossRef]
- Haahtela, T. A Biodiversity Hypothesis. Allergy 2019, 74, 1445–1456. [Google Scholar] [CrossRef]
- Wills-Karp, M.; Santeliz, J.; Karp, C.L. The Germless Theory of Allergic Disease: Revisiting the Hygiene Hypothesis. Nat. Rev. Immunol. 2001, 1, 69–75. [Google Scholar] [CrossRef]
- Van Mierlo, M.M.F.; Totté, J.E.E.; Fieten, K.B.; van den Broek, T.J.; Schuren, F.H.J.; Pardo, L.M.; Pasmans, S.G.M.A. The Influence of Treatment in Alpine and Moderate Maritime Climate on the Composition of the Skin Microbiome in Patients with Difficult to Treat Atopic Dermatitis. Clin. Exp. Allergy 2019, 49, 1437–1445. [Google Scholar] [CrossRef]
- Bosman, E.S.; Albert, A.Y.; Lui, H.; Dutz, J.P.; Vallance, B.A. Skin Exposure to Narrow Band Ultraviolet (Uvb) Light Modulates the Human Intestinal Microbiome. Front. Microbiol. 2019, 10, 477346. [Google Scholar] [CrossRef]
- Fieten, K.B.; Drijver-Messelink, M.T.; Cogo, A.; Charpin, D.; Sokolowska, M.; Agache, I.; Taborda-Barata, L.M.; Eguiluz-Gracia, I.; Braunstahl, G.J.; Seys, S.F.; et al. Alpine Altitude Climate Treatment for Severe and Uncontrolled Asthma: An EAACI Position Paper. Allergy 2022, 77, 1991. [Google Scholar] [CrossRef]
- Cunliffe, W.; Cotterill, J. The Acnes: Clinical Features, Pathogenesis and Treatment. In Major Problems in Dermatology; Saunders: Philadelphia, PA, USA, 1975; Volume 6. [Google Scholar]
- Callewaert, C.; Ravard Helffer, K.; Lebaron, P. Skin Microbiome and Its Interplay with the Environment. Am. J. Clin. Dermatol. 2020, 21, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.F.; Ming, L.C.; Wong, L.C. Dermatologic Reactions to Disinfectant Use during the COVID-19 Pandemic. Clin. Dermatol. 2021, 39, 314. [Google Scholar] [CrossRef] [PubMed]
- Goossens, A. Contact-Allergic Reactions to Cosmetics. J. Allergy 2011, 2011, 467071. [Google Scholar] [CrossRef] [PubMed]
- Salverda, J.G.W.; Bragt, P.J.C.; de Wit-Bos, L.; Rustemeyer, T.; Coenraads, P.J.; Tupker, R.A.; Kunkeler, L.C.M.; Laheij-de Boer, A.-M.; Stenveld, H.J.; van Ginkel, C.J.W.; et al. Results of a Cosmetovigilance Survey in The Netherlands. Contact Dermat. 2013, 68, 139–148. [Google Scholar] [CrossRef]
- Heisterberg, M.V.; Menné, T.; Johansen, J.D. Contact Allergy to the 26 Specific Fragrance Ingredients to Be Declared on Cosmetic Products in Accordance with the EU Cosmetics Directive. Contact Dermat. 2011, 65, 266–275. [Google Scholar] [CrossRef]
- Warshaw, E.M.; Buchholz, H.J.; Belsito, D.V.; Maibach, H.I.; Fowler, J.F.; Rietschel, R.L.; Zug, K.A.; Mathias, C.G.T.; Pratt, M.D.; Sasseville, D.; et al. Allergic Patch Test Reactions Associated with Cosmetics: Retrospective Analysis of Cross-Sectional Data from the North American Contact Dermatitis Group, 2001–2004. J. Am. Acad. Dermatol. 2009, 60, 23–38. [Google Scholar] [CrossRef]
- Berne, B.; Tammela, M.; Färm, G.; Inerot, A.; Lindberg, M. Can the Reporting of Adverse Skin Reactions to Cosmetics Be Improved? A Prospective Clinical Study Using a Structured Protocol. Contact Dermat. 2008, 58, 223–227. [Google Scholar] [CrossRef]
- Berne, B.; Boström, A.; Grahnén, A.F.; Tammela, M. Adverse Effects of Cosmetics and Toiletries Reported to the Swedish Medical Products Agency 1989–1994. Contact Dermat. 1996, 34, 359–362. [Google Scholar] [CrossRef]
- Levin, J.; Miller, R. A Guide to the Ingredients and Potential Benefits of Over-the-Counter Cleansers and Moisturizers for Rosacea Patients. J. Clin. Aesthet. Dermatol. 2011, 4, 31. [Google Scholar]
- Del Rosso, J.Q. Adjunctive Skin Care in the Management of Rosacea: Cleansers, Moisturizers, and Photoprotectants. Cutis 2005, 75 (Suppl. 3), 17–21; discussion 33. [Google Scholar]
- Jung, Y.C.; Kim, E.J.; Cho, J.C.; Suh, K.D.; Nam, G.W. Effect of Skin PH for Wrinkle Formation on Asian: Korean, Vietnamese and Singaporean. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e328–e332. [Google Scholar] [CrossRef]
- Abbas, S.; Goldberg, J.W.; Massaro, M. Personal Cleanser Technology and Clinical Performance. Dermatol. Ther. 2004, 17, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Gfatter, R.; Hackl, P.; Braun, F. Effects of Soap and Detergents on Skin Surface PH, Stratum Corneum Hydration and Fat Content in Infants. Dermatology 1997, 195, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural Skin Surface PH Is on Average below 5, Which Is Beneficial for Its Resident Flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Blum, H.E. The Human Microbiome. Adv. Med. Sci. 2017, 62, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.G.; Horswill, A.R. Overcoming PH Defenses on the Skin to Establish Infections. PLoS Pathog. 2022, 18, e1010512. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Hoptroff, M.; Arnold, D.; Eccles, R.; Campbell-Lee, S. In-Vivo Impact of Common Cosmetic Preservative Systems in Full Formulation on the Skin Microbiome. PLoS ONE 2021, 16, e0254172. [Google Scholar] [CrossRef]
- Bouslimani, A.; Da Silva, R.; Kosciolek, T.; Janssen, S.; Callewaert, C.; Amir, A.; Dorrestein, K.; Melnik, A.V.; Zaramela, L.S.; Kim, J.N.; et al. The Impact of Skin Care Products on Skin Chemistry and Microbiome Dynamics. BMC Biol. 2019, 17, 47. [Google Scholar] [CrossRef]
- Huang, Y.X.; Li, J.; Zhao, Z.X.; Zheng, B.L.; Deng, Y.X.; Shi, W.; Steinhoff, M.; Xie, H.F. Effects of Skin Care Habits on the Development of Rosacea: A Multi-Center Retrospective Case-Control Survey in Chinese Population. PLoS ONE 2020, 15, e0231078. [Google Scholar] [CrossRef]
- Kelleher, M.M.; Phillips, R.; Brown, S.J.; Cro, S.; Cornelius, V.; Carlsen, K.C.L.; Skjerven, H.O.; Rehbinder, E.M.; Lowe, A.J.; Dissanayake, E.; et al. Skin Care Interventions in Infants for Preventing Eczema and Food Allergy. Cochrane Database Syst. Rev. 2022, 2022, CD013534. [Google Scholar] [CrossRef]
- Alinia, H.; Moradi Tuchayi, S.; Farhangian, M.E.; Huang, K.E.; Taylor, S.L.; Kuo, S.; Richardson, I.; Feldman, S.R. Rosacea Patients Seeking Advice: Qualitative Analysis of Patients’ Posts on a Rosacea Support Forum. J. Dermatol. Treat. 2016, 27, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E.; Draelos, Z.; Dréno, B.; Jansen, T.; Layton, A.; Picardo, M. Rosacea—Global Diversity and Optimized Outcome: Proposed International Consensus from the Rosacea International Expert Group. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 188–200. [Google Scholar] [CrossRef]
- Raley, E.; Quirós-Alcalá, L.; Matsui, E.C. Chemical Exposures via Personal Care Products and the Disproportionate Asthma Burden among the US Black Population. J. Allergy Clin. Immunol. Pract. 2021, 9, 3290. [Google Scholar] [CrossRef] [PubMed]
- Nurmatov, U.B.; Tagiyeva, N.; Semple, S.; Devereux, G.; Sheikh, A. Volatile Organic Compounds and Risk of Asthma and Allergy: A Systematic Review. Eur. Respir. Rev. 2015, 24, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Eberle, C.E.; Sandler, D.P.; Taylor, K.W.; White, A.J. Hair Dye and Chemical Straightener Use and Breast Cancer Risk in a Large US Population of Black and White Women. Int. J. Cancer 2020, 147, 383–391. [Google Scholar] [CrossRef]
- Scherrer, M.A.R.; Rocha, V.B.; Andrade, A.R.C. Contact Dermatitis to Methylisothiazolinone. An. Bras. Dermatol. 2015, 90, 912–914. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Kim, D.-H. Effects of Cosmetics and Their Preservatives on the Growth and Composition of Human Skin Microbiota. J. Soc. Cosmet. Sci. Korea 2015, 41, 127–134. [Google Scholar] [CrossRef]
- Hass, U.; Christiansen, S.; Andersen, D.; Rosenberg, A.; Egebjerg, K.M.; Brandt, S.; Nikolov, N.G.; Holbech, H.; Morthorst, J.E. List of Endocrine Disrupting Chemicals; Danish Centre On Endocrine Disrupters: Odense, Denmark, 2017. [Google Scholar]
- Wang, Q.; Cui, S.; Zhou, L.; He, K.; Song, L.; Liang, H.; He, C. Effect of Cosmetic Chemical Preservatives on Resident Flora Isolated from Healthy Facial Skin. J. Cosmet. Dermatol. 2019, 18, 652–658. [Google Scholar] [CrossRef]
- Guerrero, D. Dermocosmetic Management of the Red Face and Rosacea. Ann. Dermatol. Venereol. 2011, 138 (Suppl. 3), S215–S218. [Google Scholar] [CrossRef]
- Steinemann, A. International Prevalence of Chemical Sensitivity, Co-Prevalences with Asthma and Autism, and Effects from Fragranced Consumer Products. Air Qual. Atmos. Health 2019, 12, 519–527. [Google Scholar] [CrossRef]
- Sarantis, H.; Naidenko, O.V.; Gray, S.; Houlihan, J. Not So Sexy: The Health Risks of Secret Chemicals in Fragrance. 2010. Available online: www.SafeCosmetics.org (accessed on 16 November 2022).
- Raghupathi, W.; Raghupathi, V. An Empirical Study of Chronic Diseases in the United States: A Visual Analytics Approach to Public Health. Int. J. Environ. Res. Public Health 2018, 15, 431. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.O.; Gillian, S. Chronic Disease in the United States: A Worsening Health and Economic Crisis. Available online: https://www.americanactionforum.org/research/chronic-disease-in-the-united-states-a-worsening-health-and-economic-crisis/ (accessed on 10 August 2023).
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Bernstein, C.N.; Vatn, M.H.; Lakatos, P.L.; Loftus, E.V.; Tysk, C.; O’Morain, C.; Moum, B.; Colombel, J.F. Geographical Variability and Environmental Risk Factors in Inflammatory Bowel Disease. Gut 2013, 62, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Lopez, A.D. Alternative Projections of Mortality and Disability by Cause 1990–2020: Global Burden of Disease Study. Lancet 1997, 349, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.; Ahlbom, A.; Magnusson, C.; Carlsson, S. Prevalence and Incidence of Diabetes in Stockholm County 1990–2010. PLoS ONE 2014, 9, e104033. [Google Scholar] [CrossRef]
- Harjutsalo, V.; Sjöberg, L.; Tuomilehto, J. Time Trends in the Incidence of Type 1 Diabetes in Finnish Children: A Cohort Study. Lancet 2008, 371, 1777–1782. [Google Scholar] [CrossRef]
- Eder, W.; Ege, M.J.; von Mutius, E. The Asthma Epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef]
- Mayr, W.T.; Pittock, S.J.; McClelland, R.L.; Jorgensen, N.W.; Noseworthy, J.H.; Rodriguez, M. Incidence and Prevalence of Multiple Sclerosis in Olmsted County, Minnesota, 1985–2000. Neurology 2003, 61, 1373–1377. [Google Scholar] [CrossRef]
- Jousilahti, P.; Haahtela, T.; Laatikainen, T.; Mäkelä, M.; Vartiaine, E. Asthma and Respiratory Allergy Prevalence Is Still Increasing among Finnish Young Adults. Eur. Respir. J. 2016, 47, 985–987. [Google Scholar] [CrossRef]
- Anderson, E.; Durstine, J.L. Physical Activity, Exercise, and Chronic Diseases: A Brief Review. Sport. Med. Health Sci. 2019, 1, 3–10. [Google Scholar] [CrossRef]
- Center for Health Statistics. Health, United States 2019; Center for Health Statistics: Hyattsville, MD, USA, 2019.
- Taylor, B.; Wadsworth, M.; Wadsworth, J.; Peckham, C. Changes in the Reported Prevalence of Childhood Eczema since the 1939–45 War. Lancet 1984, 324, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; NISC Comparative Sequence Program; et al. Temporal Shifts in the Skin Microbiome Associated with Disease Flares and Treatment in Children with Atopic Dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.R.; Newton, J.; Hippisley-Cox, J.; Sheikh, A. Trends in the Epidemiology and Prescribing of Medication for Eczema in England. J. R. Soc. Med. 2009, 102, 108–117. [Google Scholar] [CrossRef]
- Burd, R.M. Psoriasis: A General Overview. Br. J. Hosp. Med. 2006, 67, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Tang, M.L.K. The Australasian Society of Clinical Immunology and Allergy Position Statement: Summary of Allergy Prevention in Children. Med. J. Aust. 2005, 182, 464–467. [Google Scholar] [CrossRef]
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H.; ISAAC Phase Three Study Group. Worldwide Time Trends in the Prevalence of Symptoms of Asthma, Allergic Rhinoconjunctivitis, and Eczema in Childhood: ISAAC Phases One and Three Repeat Multicountry Cross-Sectional Surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Shaw, T.E.; Currie, G.P.; Koudelka, C.W.; Simpson, E.L. Eczema Prevalence in the United States: Data from the 2003 National Survey of Children’s Health. J. Investig. Dermatol. 2011, 131, 67–73. [Google Scholar] [CrossRef]
- Papadopoulos, N.G.; Agache, I.; Bavbek, S.; Bilo, B.M.; Braido, F.; Cardona, V.; Custovic, A.; de Monchy, J.; Demoly, P.; Eigenmann, P.; et al. Research Needs in Allergy: An EAACI Position Paper, in Collaboration with EFA. Clin. Transl. Allergy 2012, 2, 21. [Google Scholar] [CrossRef]
- Bajekal, M.; Primatesta, P.; Prior, G. Health Survey for England (2001); Stationery Office: London, UK, 2003. [Google Scholar]
- Jackson, K.D.; Lajeana, M.P.H.; Howie, D.; Lara, C.H.E.S.; Akinbami, J. Trends in Allergic Conditions Among Children: United States, 1997–2011. NCHS Data Brief 2013, 1–8. Available online: https://www.cdc.gov/nchs/data/databriefs/db121.pdf (accessed on 26 June 2023).
- Ng, A.E.; Boersma, P. Diagnosed Allergic Conditions in Adults: United States, 2021. NCHS Data Brief 2021. Available online: https://www.cdc.gov/nchs/products/index.htm (accessed on 27 June 2023).
- Salah, S.; Taieb, C.; Demessant, A.L.; Haftek, M. Prevalence of Skin Reactions and Self-Reported Allergies in 5 Countries with Their Social Impact Measured through Quality of Life Impairment. Int. J. Environ. Res. Public Health 2021, 18, 4501. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, H.; Koo, H.Y.R.; You, J.; Yu, D.S.; Lee, Y.B.; Lee, M. Severe Scalp Psoriasis Microbiome Has Increased Biodiversity and Relative Abundance of Pseudomonas Compared to Mild Scalp Psoriasis. J. Clin. Med. 2022, 11, 7133. [Google Scholar] [CrossRef]
- Gao, Z.; Tseng, C.; Strober, B.E.; Pei, Z.; Blaser, M.J. Substantial Alterations of the Cutaneous Bacterial Biota in Psoriatic Lesions. PLoS ONE 2008, 3, e2719. [Google Scholar] [CrossRef]
- Rainer, B.M.; Thompson, K.G.; Antonescu, C.; Florea, L.; Mongodin, E.F.; Bui, J.; Fischer, A.H.; Pasieka, H.B.; Garza, L.A.; Kang, S.; et al. Characterization and Analysis of the Skin Microbiota in Rosacea: A Case–Control Study. Am. J. Clin. Dermatol. 2020, 21, 139. [Google Scholar] [CrossRef] [PubMed]
- Perez Perez, G.I.; Gao, Z.; Jourdain, R.; Ramirez, J.; Gany, F.; Clavaud, C.; Demaude, J.; Breton, L.; Blaser, M.J. Body Site Is a More Determinant Factor than Human Population Diversity in the Healthy Skin Microbiome. PLoS ONE 2016, 11, e0151990. [Google Scholar] [CrossRef] [PubMed]
- Findley, K.; Grice, E.A. The Skin Microbiome: A Focus on Pathogens and Their Association with Skin Disease. PLoS Pathog. 2014, 10, e1004436. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, X.; Li, X.; He, X.; Xiong, X.; Lai, J. Epidermal Barrier Integrity Is Associated with Both Skin Microbiome Diversity and Composition in Patients with Acne Vulgaris. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2065–2075. [Google Scholar] [CrossRef]
- Hrestak, D.; Matijašić, M.; Paljetak, H.Č.; Drvar, D.L.; Hadžavdić, S.L.; Perić, M. Skin Microbiota in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 3503. [Google Scholar] [CrossRef]
- Byrd, A.L.; Deming, C.; Cassidy, S.K.B.; Harrison, O.J.; Ng, W.I.; Conlan, S.; Belkaid, Y.; Segre, J.A.; Kong, H.H. Staphylococcus Aureus and Staphylococcus Epidermidis Strain Diversity Underlying Pediatric Atopic Dermatitis. Sci. Transl. Med. 2017, 9, eaal4651. [Google Scholar] [CrossRef]
- Zaidi, A.K.; Spaunhurst, K.; Sprockett, D.; Thomason, Y.; Mann, M.W.; Fu, P.; Ammons, C.; Gerstenblith, M.; Tuttle, M.S.; Popkin, D.L. Characterization of the Facial Microbiome in Twins Discordant for Rosacea. Exp. Dermatol. 2018, 27, 295–298. [Google Scholar] [CrossRef]
- Yan, D.; Issa, N.; Afifi, L.; Jeon, C.; Chang, H.W.; Liao, W. The Role of the Skin and Gut Microbiome in Psoriatic Disease. Curr. Dermatol. Rep. 2017, 6, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Alekseyenko, A.V.; Perez-Perez, G.I.; De Souza, A.; Strober, B.; Gao, Z.; Bihan, M.; Li, K.; Methé, B.A.; Blaser, M.J. Community Differentiation of the Cutaneous Microbiota in Psoriasis. Microbiome 2013, 1, 31. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, M.; Vicaretti, M.; Sparks, J.; Bansal, S.; Bush, S.; Liu, M.; Darling, A.; Harry, E.; Burke, C.M. A Longitudinal Study of the Diabetic Skin and Wound Microbiome. PeerJ 2017, 5, e3543. [Google Scholar] [CrossRef] [PubMed]
- Salgado, V.R.; de Queiroz, A.T.L.; Sanabani, S.S.; de Oliveira, C.I.; Carvalho, E.M.; Costa, J.M.L.; Barral-Netto, M.; Barral, A. The Microbiological Signature of Human Cutaneous Leishmaniasis Lesions Exhibits Restricted Bacterial Diversity Compared to Healthy Skin. Mem. Inst. Oswaldo Cruz 2016, 111, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ring, H.C.; Thorsen, J.; Saunte, D.M.; Lilje, B.; Bay, L.; Riis, P.T.; Larsen, N.; Andersen, L.O.; Nielsen, H.V.; Miller, I.M.; et al. The Follicular Skin Microbiome in Patients with Hidradenitis Suppurativa and Healthy Controls. JAMA Dermatol. 2017, 153, 897–905. [Google Scholar] [CrossRef]
- Mekadim, C.; Skalnikova, H.K.; Cizkova, J.; Cizkova, V.; Palanova, A.; Horak, V.; Mrazek, J. Dysbiosis of Skin Microbiome and Gut Microbiome in Melanoma Progression. BMC Microbiol. 2022, 22, 63. [Google Scholar] [CrossRef]
- Huang, C.; Yi, X.; Long, H.; Zhang, G.; Wu, H.; Zhao, M.; Lu, Q. Disordered Cutaneous Microbiota in Systemic Lupus Erythematosus. J. Autoimmun. 2020, 108, 102391. [Google Scholar] [CrossRef]
- Schmiechen, Z.C.; Weissler, K.A.; Frischmeyer-Guerrerio, P.A. Recent Developments in Understanding the Mechanisms of Food Allergy. Curr. Opin. Pediatr. 2019, 31, 807. [Google Scholar] [CrossRef]
- Redlich, C.A. Skin Exposure and Asthma: Is There a Connection? Proc. Am. Thorac. Soc. 2010, 7, 134. [Google Scholar] [CrossRef]
- Kemter, A.M.; Nagler, C.R. Influences on Allergic Mechanisms through Gut, Lung, and Skin Microbiome Exposures. J. Clin. Investig. 2019, 129, 1483–1492. [Google Scholar] [CrossRef]
- Yaneva, M.; Darlenski, R. The Link between Atopic Dermatitis and Asthma-Immunological Imbalance and Beyond. Asthma Res. Pract. 2021, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Hojman, L.; Karsulovic, C. Cardiovascular Disease-Associated Skin Conditions. Vasc. Health Risk Manag. 2022, 18, 43. [Google Scholar] [CrossRef]
- Cowburn, A.S.; Macias, D.; Summers, C.; Chilvers, E.R.; Johnson, R.S. Cardiovascular Adaptation to Hypoxia and the Role of Peripheral Resistance. eLife 2017, 6, e28755. [Google Scholar] [CrossRef] [PubMed]
- Cotter, C.; Walsh, S. Cutaneous Sequelae of a National Health Crisis: Obesity and the Skin. Ski. Health Dis. 2021, 1, e7. [Google Scholar] [CrossRef] [PubMed]
- Redel, H.; Gao, Z.; Li, H.; Alekseyenko, A.V.; Zhou, Y.; Perez-Perez, G.I.; Weinstock, G.; Sodergren, E.; Blaser, M.J. Quantitation and Composition of Cutaneous Microbiota in Diabetic and Nondiabetic Men. J. Infect. Dis. 2013, 207, 1105–1114. [Google Scholar] [CrossRef]
- Goswami, A.; Wendt, F.R.; Pathak, G.A.; Tylee, D.S.; De Angelis, F.; De Lillo, A.; Polimanti, R. Role of Microbes in the Pathogenesis of Neuropsychiatric Disorders. Front. Neuroendocrinol. 2021, 62, 100917. [Google Scholar] [CrossRef]
- Niemann, N.; Billnitzer, A.; Jankovic, J. Parkinson’s Disease and Skin. Park. Relat. Disord. 2021, 82, 61–76. [Google Scholar] [CrossRef]
- Konig, M.F. The Microbiome in Autoimmune Rheumatic Disease. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101473. [Google Scholar] [CrossRef]
- Huang, B.L.; Chandra, S.; Shih, D.Q. Skin Manifestations of Inflammatory Bowel Disease. Front. Physiol. 2012, 3, 13. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Fagan, A.; Sikaroodi, M.; Kakiyama, G.; Takei, H.; Degefu, Y.; Pandak, W.M.; Hylemon, P.B.; Fuchs, M.; John, B.; et al. Alterations in Skin Microbiomes of Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2019, 17, 2581–2591.e15. [Google Scholar] [CrossRef]
- Wan, P.; Chen, J. A Calm, Dispassionate Look at Skin Microbiota in Atopic Dermatitis: An Integrative Literature Review. Dermatol. Ther. 2020, 10, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.H.; Oppedisano, F.; Joseph, S.J.; Boyle, R.J.; Licciardi, P.V.; Robins-Browne, R.M.; Tang, M.L.K. Reduced Gut Microbial Diversity in Early Life Is Associated with Later Development of Eczema but Not Atopy in High-Risk Infants. Pediatr. Allergy Immunol. 2012, 23, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Karlsson, C.; Olsson, C.; Adlerberth, I.; Wold, A.E.; Strachan, D.P.; Martricardi, P.M.; Åberg, N.; Perkin, M.R.; Tripodi, S.; et al. Reduced Diversity in the Early Fecal Microbiota of Infants with Atopic Eczema. J. Allergy Clin. Immunol. 2008, 121, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low Gut Microbiota Diversity in Early Infancy Precedes Asthma at School Age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef]
- Schaubeck, M.; Clavel, T.; Calasan, J.; Lagkouvardos, I.; Haange, S.B.; Jehmlich, N.; Basic, M.; Dupont, A.; Hornef, M.; Von Bergen, M.; et al. Dysbiotic Gut Microbiota Causes Transmissible Crohn’s Disease-like Ileitis Independent of Failure in Antimicrobial Defence. Gut 2016, 65, 225–237. [Google Scholar] [CrossRef]
- Williams, M.R.; Gallo, R.L. Evidence That Human Skin Microbiome Dysbiosis Promotes Atopic Dermatitis. J. Investig. Dermatol. 2017, 137, 2460. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Chen, Z.; Yau, J.W.K.; Chan, K.C.C.; Leung, A.S.Y.; Chan, O.M.; Yeung, A.C.M.; Yuen, C.L.Y.; Chan, P.K.S.; et al. Early-Life Skin Microbial Biomarkers for Eczema Phenotypes in Chinese Toddlers. Pathogens 2023, 12, 697. [Google Scholar] [CrossRef]
- Blicharz, L.; Rudnicka, L.; Czuwara, J.; Waśkiel-Burnat, A.; Goldust, M.; Olszewska, M.; Samochocki, Z. The Influence of Microbiome Dysbiosis and Bacterial Biofilms on Epidermal Barrier Function in Atopic Dermatitis—An Update. Int. J. Mol. Sci. 2021, 22, 8403. [Google Scholar] [CrossRef]
- Chen, Y.; Knight, R.; Gallo, R.L. Evolving Approaches to Profiling the Microbiome in Skin Disease. Front. Immunol. 2023, 14, 1151527. [Google Scholar] [CrossRef]
- Demehri, S.; Morimoto, M.; Holtzman, M.J.; Kopan, R. Skin-Derived TSLP Triggers Progression from Epidermal-Barrier Defects to Asthma. PLoS Biol. 2009, 7, 1000067. [Google Scholar] [CrossRef]
- Yamanaka, K.; Nakanishi, T.; Saito, H.; Maruyama, J.; Isoda, K.; Yokochi, A.; Imanaka-Yoshida, K.; Tsuda, K.; Kakeda, M.; Okamoto, R.; et al. Persistent Release of IL-1s from Skin Is Associated with Systemic Cardio-Vascular Disease, Emaciation and Systemic Amyloidosis: The Potential of Anti-IL-1 Therapy for Systemic Inflammatory Diseases. PLoS ONE 2014, 9, e104479. [Google Scholar] [CrossRef]
- Fernández-Espejo, E. Microorganisms Associated with Increased Risk of Parkinson’s Disease. Neurologia 2022, 38, 495–503. [Google Scholar] [CrossRef]
- Ravn, A.H.; Thyssen, J.P.; Egeberg, A. Skin Disorders in Parkinson’s Disease: Potential Biomarkers and Risk Factors. Clin. Cosmet. Investig. Dermatol. 2017, 10, 87–92. [Google Scholar] [CrossRef]
- Cannon, T.; Gruenheid, S. Microbes and Parkinson’s Disease: From Associations to Mechanisms. Trends Microbiol. 2022, 30, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Haikal, C.; Pascual, L.O.; Najarzadeh, Z.; Bernfur, K.; Svanbergsson, A.; Otzen, D.E.; Linse, S.; Li, J.Y. The Bacterial Amyloids Phenol Soluble Modulins from Staphylococcus aureus Catalyze Alpha-Synuclein Aggregation. Int. J. Mol. Sci. 2021, 22, 11594. [Google Scholar] [CrossRef] [PubMed]
- McFall-Ngai, M. Care for the Community. Nature 2007, 445, 153. [Google Scholar] [CrossRef] [PubMed]
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a Bacterial World, a New Imperative for the Life Sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Snitkin, E.S.; Yockey, L.J.; Bermudez, D.M.; Comparative, N.; Program, S.; Liechty, K.W.; Segre, J.A. Longitudinal Shift in Diabetic Wound Microbiota Correlates with Prolonged Skin Defense Response. Proc. Natl. Acad. Sci. USA 2010, 33107, 14799–14804. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Chinthrajah, R.S.; Hernandez, J.D.; Boyd, S.D.; Galli, S.J.; Nadeau, K.C. Molecular and Cellular Mechanisms of Food Allergy and Food Tolerance. J. Allergy Clin. Immunol. 2016, 137, 984. [Google Scholar] [CrossRef]
- Nedorost, S.; Hammond, M. Art of Prevention: Allergic Sensitization through Damaged Skin: Atopic, Occupational, and Stasis Dermatitis. Int. J. Womens Dermatol. 2020, 6, 381. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Wu, L.; Xiao, S.; Ji, Y.; Tan, Y.; Jiang, C.; Zhang, G. Dysregulation of the Gut-Brain-Skin Axis and Key Overlapping Inflammatory and Immune Mechanisms of Psoriasis and Depression. Biomed. Pharmacother. 2021, 137, 111065. [Google Scholar] [CrossRef] [PubMed]
- Korman, N.; Korman, N.; Squibb, B.-M.; Lilly, E. Management of Psoriasis as a Systemic Disease: What Is the Evidence? Br. J. Dermatol. 2020, 182, 840–848. [Google Scholar] [CrossRef]
- Nguyen, V.A.T.; Vural, D.C. Theoretical Guidelines for Editing Ecological Communities. J. Theor. Biol. 2022, 534, 110945. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Gallo, R.L. Antimicrobial Peptides in Human Skin Disease. Eur. J. Dermatol. 2008, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Herman, A.P. Antimicrobial Peptides Activity in the Skin. Ski. Res. Technol. 2019, 25, 111–117. [Google Scholar] [CrossRef]
- Rademacher, F.; Gläser, R.; Harder, J. Antimicrobial Peptides and Proteins: Interaction with the Skin Microbiota. Exp. Dermatol. 2021, 30, 1496–1508. [Google Scholar] [CrossRef]
- Dahlhoff, M.; Zouboulis, C.C.; Schneider, M.R. Expression of Dermcidin in Sebocytes Supports a Role for Sebum in the Constitutive Innate Defense of Human Skin. J. Dermatol. Sci. 2016, 81, 124–126. [Google Scholar] [CrossRef]
- Noble, W.C. Staphylococci on the Skin. In The Skin Microflora and Microbial Skin Disease; Noble, W.C., Ed.; Cambridge University Press: London, UK, 2004; pp. 135–152. [Google Scholar]
- Battaglia, M.; Garrett-Sinha, L.A. Bacterial Infections in Lupus: Roles in Promoting Immune Activation and in Pathogenesis of the Disease. J. Transl. Autoimmun. 2021, 4, 100078. [Google Scholar] [CrossRef]
- Roe, D. Biodiversity Loss—More than an Environmental Emergency. Lancet Planet Health 2019, 3, e287–e289. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Byrnes, J.E.K.; Isbell, F.; Gamfeldt, L.; Griffin, J.N.; Eisenhauer, N.; Hensel, M.J.S.; Hector, A.; Cardinale, B.J.; Duffy, J.E. Biodiversity Enhances Ecosystem Multifunctionality across Trophic Levels and Habitats. Nat. Commun. 2015, 6, 6936. [Google Scholar] [CrossRef] [PubMed]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; He, J.-S.; Nakashizuka, T.; Raffaelli, D.; Schmid, B. Quantifying the Evidence for Biodiversity Effects on Ecosystem Functioning and Services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Gamfeldt, L.; Hillebrand, H.; Jonsson, P.R. Multiple Functions Increase the Importance of Biodiversity for Overall Ecosystem Functioning. Ecology 2008, 89, 1223–1231. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of Biodiversity on Ecosystem Functioning: A Consensus of Current Knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Shea, K.; Chesson, P. Community Ecology Theory as a Framework for Biological Invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Hautier, Y.; Tilman, D.; Isbell, F.; Seabloom, E.W.; Borer, E.T.; Reich, P.B. Anthropogenic Environmental Changes Affect Ecosystem Stability via Biodiversity. Science 2015, 348, 336–340. [Google Scholar] [CrossRef]
- Isbell, F.; Craven, D.; Connolly, J.; Loreau, M.; Schmid, B.; Beierkuhnlein, C.; Bezemer, T.M.; Bonin, C.; Bruelheide, H.; de Luca, E.; et al. Biodiversity Increases the Resistance of Ecosystem Productivity to Climate Extremes. Nature 2015, 526, 574–577. [Google Scholar] [CrossRef]
- Sherwin, W.B.; Fornells, N.P.I. The Introduction of Entropy and Information Methods to Ecology by Ramon Margalef. Entropy 2019, 21, 794. [Google Scholar] [CrossRef]
- Roach, T.N.F. Use and Abuse of Entropy in Biology: A Case for Caliber. Entropy 2020, 22, 1335. [Google Scholar] [CrossRef] [PubMed]
- Spellerberg, I.F.; Fedor, P.J. A Tribute to Claude Shannon (1916–2001) and a Plea for More Rigorous Use of Species Richness, Species Diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- De Keersmaecker, W.; Lhermitte, S.; Honnay, O.; Farifteh, J.; Somers, B.; Coppin, P. How to Measure Ecosystem Stability? An Evaluation of the Reliability of Stability Metrics Based on Remote Sensing Time Series across the Major Global Ecosystems. Glob. Chang Biol. 2014, 20, 2149–2161. [Google Scholar] [CrossRef]
- Finlay, B.B.; Arrieta, M.-C. Let Them Eat Dirt. Saving Your Child from an Oversanitized World; Windmill Books: London, UK, 2016. [Google Scholar]
- Isbell, F.; Hector, A.; Loreau, M. Large-Scale Biodiversity Experiments. In Encyclopedia of Biodiversity; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Pongsiri, M.J.; Roman, J.; Ezenwa, V.O.; Goldberg, T.L.; Koren, H.S.; Newbold, S.C.; Ostfeld, R.S.; Pattanayak, S.K.; Salkeld, D.J. Biodiversity Loss Affects Global Disease Ecology. Bioscience 2009, 59, 945–954. [Google Scholar] [CrossRef]
- Davis, A.P.; Chadburn, H.; Moat, J.; O’Sullivan, R.; Hargreaves, S.; Lughadha, E.N. High Extinction Risk for Wild Coffee Species and Implications for Coffee Sector Sustainability. Sci. Adv. 2019, 5, 3473–3489. [Google Scholar] [CrossRef]
- Dainese, M.; Martin, E.A.; Aizen, M.A.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Carvalheiro, L.G.; Chaplin-Kramer, R.; Gagic, V.; Garibaldi, L.A.; et al. A Global Synthesis Reveals Biodiversity-Mediated Benefits for Crop Production. Sci. Adv. 2019, 5, eaax0121. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Wallen-Russell, C.; Wallen-Russell, S. Topical Probiotics Do Not Satisfy New Criteria for Effective Use Due to Insufficient Skin Microbiome Knowledge. Cosmetics 2021, 8, 90. [Google Scholar] [CrossRef]
- Dotterud, C.K.; Avershina, E.; Sekelja, M.; Simpson, M.R.; Rudi, K.; Storrø, O.; Johnsen, R.; Eien, T. Does Maternal Perinatal Probiotic Supplementation Alter the Intestinal Microbiota of Mother and Child? J. Pediatr. Gastroenterol. Nutr. 2015, 61, 200–207. [Google Scholar] [CrossRef]
- França, K. Topical Probiotics in Dermatological Therapy and Skincare: A Concise Review. Dermatol. Ther. 2020, 11, 71–77. [Google Scholar] [CrossRef]
- Lee, G.R.; Maarouf, M.; Hendricks, A.J.; Lee, D.E.; Shi, V.Y. Topical Probiotics: The Unknowns behind Their Rising Popularity. Dermatol. Online J. 2019, 25, 5–6. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health Benefits of Probiotics: A Review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L. Probiotic Use in Clinical Practice: What Are the Risks? Am. J. Clin. Nutr. 2006, 83, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Björkstén, B.; Engstrand, L.; Andersson, A.F. Decreased Gut Microbiota Diversity, Delayed Bacteroidetes Colonisation and Reduced Th1 Responses in Infants Delivered by Caesarean Section. Gut 2014, 63, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Zahniser, A.; Singh, A. Return of the Wolves to Yellowstone National Park, USA: A Model of Ecosystem Restoration. Biodiversity 2004, 5, 3–7. [Google Scholar] [CrossRef]
- Glaser, B. Prehistorically Modified Soils of Central Amazonia: A Model for Sustainable Agriculture in the Twenty-First Century. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 187. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Kao, M.C.; Zhang, L.; Zouboulis, C.C.; Gallo, R.L.; Huang, C.-M. Sebum Free Fatty Acids Enhance the Innate Immune Defense of Human Sebocytes by Upregulating β-Defensin-2 Expression. J. Investig. Dermatol. 2010, 130, 985–994. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Kao, J.; Ahn, S.K.; Feingold, K.R.; Elias, P.M.; Jain, M. Generation of Free Fatty Acids from Phospholipids Regulates Stratum Corneum Acidification and Integrity. J. Investig. Dermatol. 2001, 117, 44–51. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef]
- Chen, J.; Siliceo, S.L.; Ni, Y.; Nielsen, H.B.; Xu, A.; Panagiotou, G. Identification of Robust and Generalizable Biomarkers for Microbiome-Based Stratification in Lifestyle Interventions. Microbiome 2023, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Munsch, S.H.; Andrews, K.S.; Crozier, L.G.; Fonner, R.; Gosselin, J.L.; Greene, C.M.; Harvey, C.J.; Lundin, J.I.; Pess, G.R.; Samhouri, J.F.; et al. Potential for Ecological Nonlinearities and Thresholds to Inform Pacific Salmon Management. Ecosphere 2020, 11, e03302. [Google Scholar] [CrossRef]
- Myers, S.S.; Gaffikin, L.; Golden, C.D.; Ostfeld, R.S.; Redford, K.H.; Ricketts, T.H.; Turner, W.R.; Osofsky, S.A. Human Health Impacts of Ecosystem Alteration. Proc. Natl. Acad. Sci. USA 2013, 110, 18753–18760. [Google Scholar] [CrossRef] [PubMed]
- Lenton, T.M.; van Oijen, M. Gaia as a Complex Adaptive System. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 683. [Google Scholar] [CrossRef]
- Oestreicher, C. A History of Chaos Theory. Dialogues Clin. Neurosci. 2007, 9, 279. [Google Scholar] [CrossRef]
- Ghys, É. The Butterfly Effect. In Proceedings of the 12th International Congress on Mathematical Education; Springer: Berlin/Heidelberg, Germany, 2015; pp. 19–39. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallen-Russell, C.; Pearlman, N.; Wallen-Russell, S.; Cretoiu, D.; Thompson, D.C.; Voinea, S.C. A Catastrophic Biodiversity Loss in the Environment Is Being Replicated on the Skin Microbiome: Is This a Major Contributor to the Chronic Disease Epidemic? Microorganisms 2023, 11, 2784. https://doi.org/10.3390/microorganisms11112784
Wallen-Russell C, Pearlman N, Wallen-Russell S, Cretoiu D, Thompson DC, Voinea SC. A Catastrophic Biodiversity Loss in the Environment Is Being Replicated on the Skin Microbiome: Is This a Major Contributor to the Chronic Disease Epidemic? Microorganisms. 2023; 11(11):2784. https://doi.org/10.3390/microorganisms11112784
Chicago/Turabian StyleWallen-Russell, Christopher, Nancy Pearlman, Samuel Wallen-Russell, Dragos Cretoiu, Dana Claudia Thompson, and Silviu Cristian Voinea. 2023. "A Catastrophic Biodiversity Loss in the Environment Is Being Replicated on the Skin Microbiome: Is This a Major Contributor to the Chronic Disease Epidemic?" Microorganisms 11, no. 11: 2784. https://doi.org/10.3390/microorganisms11112784