Serological Assays for Alveolar and Cystic Echinococcosis—A Comparative Multi-Test Study in Switzerland and Kyrgyzstan
Abstract
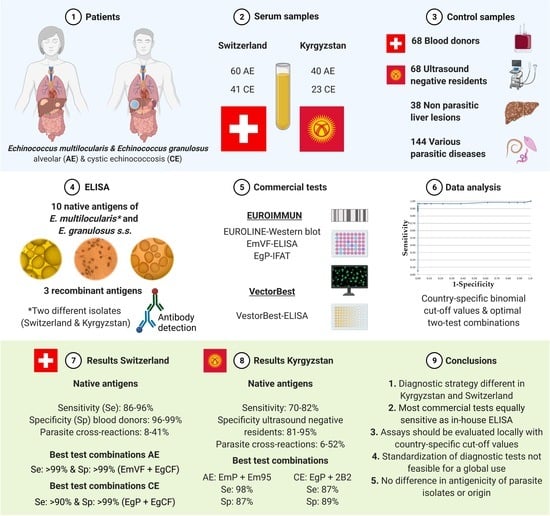
:1. Introduction
2. Material and Methods
2.1. Serum Samples of AE and CE Patients
| Patient’s Characteristics | Swiss AE | Kyrgyz AE | Swiss CE | Kyrgyz CE |
|---|---|---|---|---|
| Number of patients | N = 60 | N = 40 | N = 41 | N = 23 |
| Males | 22 | 18 | 22 | 11 |
| Females | 38 | 22 | 19 | 12 |
| Average age males | 58 (18–79) | 32 (9–65) | 38 (20–66) | 38 (23–73) |
| Average age females | 54 (21–72) | 28 (7–57) | 41 (12–60) | 36 (17–64) |
| WHO classification/staging * | P1N0M0: 18 | P1N0M0: 0 | CE1: 11 | CE1: 12 |
| P2N0M0: 11 | P2N0M0: 6 | CE2: 10 | CE2: 7 | |
| P3N0M0: 5 | P3N0M0: 6 | CE3: 8 | CE3: 3 | |
| P4N0M0: 1 | P4N0M0: 2 | CE4: 3 | CE4: 1 | |
| PXN1MX: 18 | PXN1MX: 6 | CE5: 2 | CE5: 0 | |
| AE & CE: M1/disseminated | PXNXM1: 11 | PXNXM1: 1 | 4 | 0 |
| N/A: not available | N/A: 0 | N/A: 20 | N/A: 3 | N/A: 0 |
| Lesion size (cm) | ||||
| 0.5–2.5 | 10 | 1 | 1 | 0 |
| 2.5–5.0 | 18 | 2 | 12 | 3 |
| 5.0–7.5 | 11 | 9 | 8 | 8 |
| 7.5–10.0 | 6 | 5 | 7 | 9 |
| >10.0 | 15 | 7 | 8 | 3 |
| N/A | 0 | 16 | 5 | 0 |
| Total IgE | ||||
| pre-treatment data avaliable | 56/60 | N/A | 37/41 | N/A |
| average IgE values | 1262 kU/L | N/A | 493 kU/L | N/A |
| median IgE values | 159 kU/L | N/A | 110 kU/L | N/A |
| elevated IgE values (>100 kU/L) | 32 (57%) | N/A | 20 (54%) | N/A |
| Lymphocytes | ||||
| pre-treatment data avaliable | 56/60 | N/A | 37/41 | N/A |
| average lymphocytes ×103/µL | 1.69 | N/A | 1.98 | N/A |
| median lymphocytes ×103/µL | 1.73 | N/A | 1.85 | N/A |
| Lymphopenia (<1.50 × 103/µL) | 21 (38%) | N/A | 7 (19%) | N/A |
| Elevated IgE and/or lymphopenia | 40 (71%) | N/A | 24 (65%) | N/A |
2.2. Serum Samples for Evaluating Cut-off Values and Cross-Reactions
2.3. Parasite Isolates
2.3.1. E. multilocularis European Isolate J2012
2.3.2. E. multilocularis Asian Isolate AT17
2.3.3. E. granulosus sensu stricto (s.s.) Sheep Isolate
2.4. Antigens & Commercial Tests
2.4.1. In Vitro-Produced Vesicle Crude Antigens of E. multilocularis & E. granulosus s.s.
2.4.2. Antigen on-Plate Purification with mAb Em2G11 and mAb EmG3
2.4.3. recEm95 Antigen
2.4.4. Commercial Tests
2.4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Statistical Analysis & Evaluation of Test Performances
2.6. Excluded Diagnostic Assays
3. Results
3.1. General Remarks
| ELISA */Commercial Test ** | Origin | Remarks | Citation or Material & Methods |
|---|---|---|---|
| EmVF CH/KG * | E. multilocularis | in vitro vesicle fluid | Schweiger et al., 2011 [20] |
| EmVC CH/KG * | E. multilocularis | in vitro vesicle crude antigen | 2.4.1 Vesicle crude antigens |
| EmP CH/KG* | E. multilocularis | protoscolex crude antigen from Meriones | Schweiger et al., 2011 [20] |
| Em2G11 CH/KG* | E. multilocularis | affinity-purified Em2G11 antigen from EmVC | Schweiger et al., 2011 [20] |
| mAb EmG3-EmVC CH/KG* | E. multilocularis | on-plate purification with monoclonal antibody | 2.4.2 On-plate purification |
| recEm18 * | recombinant | recombinant Em18 antigen | Schweiger et al., 2011 [20] |
| recEm95 * | recombinant | recombinant Em95 antigen | Gauci et al., 2002 [35] |
| EgVC * | E. granulosus s.s. | in vitro vesicle crude antigen | 2.4.1 Vesicle crude antigens |
| mAb EmG3-EgVC * | E. granulosus s.s. | on-plate purification with monoclonal antibody | 2.4.2 On-plate purification |
| EgCF * | E. granulosus s.s. | cyst fluid from sheep | Schweiger et al., 2011 [20] |
| EgP * | E. granulosus s.s. | protoscolex crude antigen from sheep | Schweiger et al., 2011 [20] |
| EgAgB * | E. granulosus s.s. | Antigen B from cyst fluid from sheep | Schweiger et al., 2011 [20] |
| recEg2B2 * | recombinant | recombinant 2B2 antigen | Hernández-González et al., 2012 [38] |
| Western blot-species ** | EUROIMMUN AG | for species diagnosis (AE vs. CE) | 2.4.4 Commercial Tests |
| Western blot-genus ** | EUROIMMUN AG | for genus diagnosis (AE or CE) | 2.4.4 Commercial Tests |
| EmVF-ELISA ** | EUROIMMUN AG | E. multilocularis vesicle fluid ELISA | 2.4.4 Commercial Tests |
| EgP-IFAT ** | EUROIMMUN AG | E. granulosus s.s. protoscolices IFAT | 2.4.4 Commercial Tests |
| VectorBest-ELISA ** | VectorBest | Echinococcus-IgG-EIA-BEST | 2.4.4 Commercial Tests |
3.2. Alveolar Echinococcosis, Diagnostic Performance of Single Tests
| Switzerland | Kyrgyzstan | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of sera | 60 | 68 | 38 | 144 | 64 | McNemar’s Test: p values < 0.05 are significant (N/S = not significant) | 40 | 68 | 144 | 64 | |||||||||||||||||
| Antigens & Methods | Sensitivity AE patients | Specificity blood donors | Specificity NPLL | Cross-reactions parasites | Cross-reactions CE | EmVF-ELISA * | EmVC-ELISA * | EmP-ELISA * | Em2G11-ELISA * | EmG3-EmVC-ELISA * | recEm18-ELISA | recEm95-ELISA | EmG3-EgVC-ELISA | EgVC-ELISA | EgCF-ELISA | EgP-ELISA | EgAgB-ELISA | recEg2B2-ELISA | Western blot-genus ** | Western blot-species ** | EmVF-ELISA ** | EgP-IFAT ** | VectorBest-ELISA ** | Sensitivity AE patients | Specificity US negative | Cross-reactions parasites | Cross-reactions CE |
| EmVF-ELISA * | 0.96 | 0.99 | 0.95 | 0.19 | 0.86 | N/S | N/S | 0.00 | N/S | N/S | 0.00 | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | 0.74 | 0.89 | 0.11 | 0.68 | |
| EmVC-ELISA * | 0.95 | 0.99 | 0.97 | 0.19 | 0.67 | N/S | N/S | 0.02 | N/S | 0.03 | 0.02 | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | 0.82 | 0.91 | 0.15 | 0.64 | |
| EmP-ELISA * | 0.96 | 0.98 | 0.95 | 0.08 | 0.59 | N/S | N/S | 0.02 | N/S | 0.03 | 0.02 | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | 0.82 | 0.95 | 0.10 | 0.61 | |
| Em2G11-ELISA * | 0.91 | 0.97 | 1.00 | 0.29 | 0.53 | N/S | N/S | N/S | 0.01 | 0.00 | N/S | N/S | 0.01 | 0.02 | 0.02 | 0.01 | N/S | 0.02 | 0.00 | 0.02 | N/S | 0.03 | 0.80 | 0.88 | 0.38 | 0.56 | |
| EmG3-EmVC-ELISA * | 0.96 | 0.98 | 0.97 | 0.17 | 0.66 | N/S | N/S | N/S | N/S | 0.02 | 0.05 | N/S | N/S | N/S | N/S | N/S | N/S | N/S | 0.03 | N/S | N/S | N/S | 0.78 | 0.91 | 0.19 | 0.69 | |
| recEm18-ELISA | 0.82 | 0.96 | 0.95 | 0.06 | 0.13 | N/S | 0.01 | 0.02 | 0.03 | 0.02 | 0.00 | N/S | N/S | 0.03 | N/S | N/S | N/S | N/S | N/S | N/S | 0.02 | N/S | 0.70 | 0.89 | 0.06 | 0.13 | |
| recEm95-ELISA | 0.67 | 0.91 | 0.92 | 0.28 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.02 | 0.00 | 0.03 | 0.05 | 0.01 | 0.82 | 0.95 | 0.52 | 0.25 | |
| EmG3-EgVC-ELISA | 0.78 | 0.83 | 0.86 | 0.22 | 0.66 | N/S | N/S | N/S | N/S | N/S | N/S | 0.00 | N/S | N/S | N/S | N/S | N/S | N/S | 0.02 | N/S | N/S | N/S | 0.78 | 0.83 | 0.22 | 0.66 | |
| EgVC-ELISA | 0.75 | 0.83 | 0.92 | 0.19 | 0.53 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | N/S | N/S | 0.00 | N/S | N/S | N/S | N/S | N/S | 0.04 | N/S | N/S | 0.01 | 0.73 | 0.67 | 0.22 | 0.41 | |
| EgCF-ELISA | 0.95 | 0.99 | 0.97 | 0.38 | 0.86 | N/S | N/S | N/S | N/S | N/S | N/S | 0.00 | N/S | 0.00 | N/S | N/S | N/S | N/S | 0.02 | N/S | N/S | N/S | 0.76 | 0.88 | 0.29 | 0.73 | |
| EgP-ELISA | 0.96 | 0.99 | 0.95 | 0.26 | 0.83 | N/S | N/S | N/S | N/S | N/S | N/S | 0.00 | N/S | 0.00 | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | 0.77 | 0.92 | 0.16 | 0.83 | |
| EgAgB-ELISA | 0.89 | 0.98 | 0.95 | 0.35 | 0.83 | N/S | 0.03 | N/S | N/S | N/S | N/S | 0.00 | N/S | N/S | N/S | N/S | N/S | N/S | 0.02 | N/S | N/S | N/S | 0.70 | 0.81 | 0.40 | 0.84 | |
| recEg2B2-ELISA | 0.64 | 0.64 | 0.84 | 0.41 | 0.58 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | N/S | 0.00 | N/S | 0.00 | 0.00 | 0.00 | N/S | 0.04 | N/S | N/S | N/S | 0.65 | 0.65 | 0.40 | 0.84 | |
| Western blot-genus ** | 0.93 | NA | 0.97 | 0.10 | 0.83 | N/S | N/S | N/S | N/S | N/S | 0.03 | 0.00 | N/S | 0.00 | N/S | N/S | N/S | 0.00 | 0.03 | N/S | N/S | N/S | 0.65 | NA | 0.10 | 0.65 | |
| Western blot-species ** | 0.58 | NA | 0.97 | 0.11 | 0.56 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | N/S | 0.00 | N/S | 0.00 | 0.00 | 0.00 | N/S | 0.00 | N/S | 0.01 | N/S | 0.45 | NA | 0.11 | 0.48 | |
| EmVF-ELISA ** | 0.88 | NA | 1.00 | 0.18 | 0.78 | N/S | N/S | N/S | N/S | N/S | N/S | 0.00 | N/S | 0.04 | N/S | N/S | N/S | 0.01 | N/S | 0.01 | N/S | N/S | 0.55 | NA | 0.18 | 0.52 | |
| EgP-IFAT ** | 0.90 | NA | 0.97 | 0.28 | 0.83 | N/S | N/S | N/S | N/S | N/S | N/S | 0.00 | N/S | 0.00 | N/S | N/S | N/S | 0.01 | N/S | 0.01 | N/S | N/S | 0.68 | NA | 0.28 | 0.74 | |
| VectorBest-ELISA ** | 0.77 | NA | 1.00 | 0.16 | 0.63 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | N/S | N/S | 0.01 | N/S | 0.00 | 0.00 | 0.02 | N/S | 0.00 | N/S | 0.02 | 0.01 | 0.45 | NA | 0.16 | 0.35 | |
3.2.1. Subclinical Alveolar Echinococcosis, Diagnostic Performance of Single Tests
3.2.2. Alveolar Echinococcosis, Confirmation Tests
| Sensitivity AE Patients | Specificity Blood Donors | Specificity NPLL Patients | Cross-Reactions Parasitic Infections | Cross-Reactions CE Patients | |
|---|---|---|---|---|---|
| Number of Sera | 60 | 68 | 38 | 144 | 64 |
| Switzerland | |||||
| Antigens for ELISA | |||||
| EmVC * | 0.87 (0.75–0.94) | 1.00 (0.94–1.00) | 0.97 (0.86–0.99) | 0.01 (0.00–0.04) | 0.20 (0.11–0.32) |
| mAb EmG3-EmVC * | 0.87 (0.75–0.94) | 1.00 (0.94–1.00) | 0.97 (0.86–0.99) | 0.02 (0.00–0.06) | 0.34 (0.23–0.47) |
| Em2G11 * | 0.77 (0.64–0.87) | 1.00 (0.94–1.00) | 1.00 (0.91–1.00) | 0.03 (0.00–0.07) | 0.17 (0.09–0.29) |
| mAb Em2G11-EmVC * | 0.77 (0.64–0.87) | 1.00 (0.94–1.00) | 1.00 (0.91–1.00) | 0.02 (0.00–0.06) | 0.20 (0.11–0.32) |
| EmP * | 0.90 (0.79–0.96) | 1.00 (0.94–1.00) | 0.97 (0.86–0.99) | 0.03 (0.00–0.07) | 0.51 (0.39–0.64) |
| EmVF * | 0.87 (0.75–0.94) | 1.00 (0.94–1.00) | 0.97 (0.86–0.99) | 0.11 (0.06–0.17) | 0.66 (0.53–0.77) |
| recEm18 | 0.70 (0.57–0.81) | 1.00 (0.94–1.00) | 0.97 (0.86–0.99) | 0.03 (0.00–0.07) | 0.12 (0.06–0.23) |
| recEm95 | 0.45 (0.31–0.58) | 1.00 (0.94–1.00) | 0.95 (0.82–0.99) | 0.08 (0.04–0.14) | 0.02 (0.00–0.08) |
| Number of Sera | 40 | 68 | 38 | 144 | 64 |
| Kyrgyzstan | |||||
| Antigens for ELISA | |||||
| EmVC * | 0.63 (0.46–0.77) | 0.97 (0.90–0.99) | N/A | 0.11 (0.06–0.17) | 0.52 (0.39–0.64) |
| mAb EmG3-EmVC * | 0.58 (0.41–0.73) | 0.99 (0.92–0.99) | N/A | 0.02 (0.00–0.06) | 0.35 (0.23–0.47) |
| Em2G11 * | 0.50 (0.34–0.66) | 0.99 (0.92–0.99) | N/A | 0.07 (0.03–0.12) | 0.17 (0.09–0.29) |
| mAb Em2G11-EmVC * | 0.50 (0.34–0.66) | 0.99 (0.92–0.99) | N/A | 0.02 (0.00–0.06) | 0.22 (0.15–0.40) |
| EmP * | 0.63 (0.46–0.77) | 0.99 (0.92–0.99) | N/A | 0.05 (0.02–0.10) | 0.52 (0.39–0.64) |
| EmVF * | 0.55 (0.38–0.71) | 0.91 (0.82–0.97) | N/A | 0.11 (0.06–0.17) | 0.66 (0.53–0.77) |
| recEm18 | 0.45 (0.29–0.62) | 0.99 (0.92–0.99) | N/A | 0.03 (0.00–0.07) | 0.00 (0.00–0.06) |
| recEm95 | 0.58 (0.41–0.73) | 1.00 (0.94–1.00) | N/A | 0.06 (0.03–0.12) | 0.09 (0.04–0.19) |
3.3. Cystic echinococcosis, Diagnostic Performance of Single Tests
| Switzerland | Kyrgyzstan | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Sera | 41 | 68 | 38 | 144 | 100 | McNemar’s Test: p values < 0.05 are significant (N/S = not significant) | 23 | 68 | 144 | 100 | ||||||||||||||
| Antigens & Methods | Sensitivity CE patients | Specificity blood donors | Specificity NPLL | Cross-reactions parasites | Cross-reactions AE | EmVF-ELISA * | EmVC-ELISA * | EmP-ELISA * | EmG3-EmVC-ELISA * | EmG3-EgVC-ELISA | EgVC-ELISA | EgCF-ELISA | EgP-ELISA | EgAgB-ELISA | recEg2B2-ELISA | Western blot-genus ** | Western blot-species ** | EmVF-ELISA ** | EgP-IFAT ** | VectorBest-ELISA ** | Sensitivity CE patients | Specificity US negative | Cross-reactions parasites | Cross-reactions AE |
| EmVF-ELISA * | 0.94 | 0.97 | 0.95 | 0.34 | 0.87 | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | 0.00 | N/S | 0.03 | N/S | N/S | N/S | 0.70 | 0.85 | 0.14 | 0.76 | |
| EmVC-ELISA * | 0.81 | 0.89 | 0.95 | 0.35 | 0.92 | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | 0.00 | N/S | N/S | N/S | N/S | N/S | 0.66 | 0.86 | 0.28 | 0.90 | |
| EmP-ELISA * | 0.84 | 0.96 | 0.92 | 0.26 | 0.83 | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | 0.00 | N/S | N/S | N/S | N/S | N/S | 0.76 | 0.94 | 0.26 | 0.83 | |
| EmG3-EmVC-ELISA * | 0.84 | 0.93 | 0.97 | 0.23 | 0.88 | N/S | N/S | N/S | N/S | N/S | N/S | N/S | N/S | 0.01 | N/S | N/S | N/S | N/S | N/S | 0.67 | 0.88 | 0.29 | 0.86 | |
| EmG3-EgVC-ELISA | 0.82 | 0.90 | 0.82 | 0.31 | 0.85 | 0.00 | 0.04 | N/S | 0.03 | 0.02 | 0.03 | N/S | N/S | 0.00 | N/S | 0.02 | 0.03 | N/S | N/S | 0.67 | 0.78 | 0.27 | 0.83 | |
| EgVC-ELISA | 0.61 | 0.76 | 0.84 | 0.33 | 0.79 | 0.00 | 0.01 | 0.01 | 0.00 | N/S | N/S | 0.04 | N/S | N/S | N/S | N/S | N/S | N/S | N/S | 0.42 | 0.80 | 0.12 | 0.57 | |
| EgCF-ELISA | 0.93 | 0.97 | 0.97 | 0.43 | 0.91 | N/S | N/S | N/S | N/S | N/S | 0.00 | N/S | N/S | 0.02 | N/S | N/S | N/S | N/S | N/S | 0.68 | 0.88 | 0.29 | 0.81 | |
| EgP-ELISA | 0.93 | 0.97 | 0.92 | 0.32 | 0.86 | N/S | N/S | N/S | N/S | 0.04 | 0.00 | N/S | N/S | 0.00 | N/S | 0.02 | 0.03 | N/S | N/S | 0.76 | 0.93 | 0.17 | 0.78 | |
| EgAgB-ELISA | 0.86 | 0.96 | 0.92 | 0.39 | 0.79 | N/S | N/S | N/S | N/S | 0.04 | 0.00 | N/S | N/S | 0.02 | N/S | N/S | N/S | N/S | N/S | 0.68 | 0.92 | 0.34 | 0.73 | |
| recEg2B2-ELISA | 0.49 | 0.84 | 0.92 | 0.08 | 0.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | N/S | 0.00 | 0.00 | 0.00 | 0.01 | N/S | N/S | 0.01 | N/S | 0.45 | 0.86 | 0.08 | 0.33 | |
| Western blot-genus ** | 0.83 | N/A | 0.97 | 0.10 | 0.93 | N/S | N/S | N/S | N/S | N/S | 0.00 | N/S | N/S | N/S | 0.00 | N/S | N/S | N/S | N/S | 0.65 | N/A | 0.10 | 0.65 | |
| Western blot-species ** | 0.56 | N/A | 0.97 | 0.11 | 0.58 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | N/S | 0.00 | 0.00 | 0.00 | N/S | 0.00 | N/S | N/S | N/S | 0.48 | N/A | 0.11 | 0.45 | |
| EmVF-ELISA ** | 0.78 | N/A | 1.00 | 0.18 | 0.88 | N/S | N/S | N/S | N/S | N/S | 0.01 | N/S | N/S | N/S | 0.00 | N/S | 0.01 | N/S | N/S | 0.52 | N/A | 0.18 | 0.55 | |
| EgP-IFAT ** | 0.83 | N/A | 0.97 | 0.28 | 0.90 | N/S | N/S | N/S | N/S | N/S | 0.00 | N/S | N/S | N/S | 0.00 | N/S | 0.00 | N/S | N/S | 0.74 | N/A | 0.28 | 0.68 | |
| VectorBest-ELISA ** | 0.63 | N/A | 1.00 | 0.16 | 0.77 | 0.02 | 0.02 | N/S | 0.03 | N/S | N/S | 0.03 | 0.02 | 0.02 | 0.02 | N/S | N/S | N/S | 0.03 | 0.35 | N/A | 0.16 | 0.45 | |
3.4. Cross-Reactions of Patients with Non-Echinococcus Parasitic Infections for the Serological Diagnosis of Alveolar Echinococcosis
3.5. Cross-Reactions of Patients with non-Echinococcus Parasitic Infections for the Serological Diagnosis of Cystic Echinococcosis
3.6. Diagnostic Performance of Test Combinations for the Serological Diagnosis of Alveolar Echinococcosis
3.7. Diagnostic Performance of Test Combinations for the Serological Diagnosis of Cystic Echinococcosis
4. Discussion
5. Conclusions and Practical Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eckert, J.; Deplazes, P. Biological, Epidemiological, and Clinical Aspects of Echinococcosis, a Zoonosis of Increasing Concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, P.; Menezes da Silva, A.; Akhan, O.; Müllhaupt, B.; Vizcaychipi, K.A.; Budke, C.; Vuitton, D.A. The Echinococcoses: Diagnosis, Clinical Management and Burden of Disease. Adv. Parasitol. 2017, 96, 259–369. [Google Scholar] [PubMed]
- Romig, T.; Deplazes, P.; Jenkins, D.; Giraudoux, P.; Massolo, A.; Craig, P.S.; Wassermann, M.; Takahashi, K.; de la Rue, M. Chapter Five—Ecology and Life Cycle Patterns of Echinococcus Species. In Echinococcus and Echinococcosis, Part A; Advances in Parasitology; Thompson, R.C.A., Deplazes, P., Lymbery, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 95, pp. 213–314. [Google Scholar]
- Deplazes, P.; Rinaldi, L.; Alvarez Rojas, C.A.; Torgerson, P.R.; Harandi, M.F.; Romig, T.; Antolova, D.; Schurer, J.M.; Lahmar, S.; Cringoli, G.; et al. Global Distribution of Alveolar and Cystic Echinococcosis. Adv. Parasitol. 2017, 95, 315–493. [Google Scholar] [PubMed] [Green Version]
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Fèvre, E.M.; Sripa, B.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vuitton, D.A.; Demonmerot, F.; Knapp, J.; Richou, C.; Grenouillet, F.; Chauchet, A.; Vuitton, L.; Bresson-Hadni, S.; Millon, L. Clinical epidemiology of human AE in Europe. Vet. Parasitol. 2015, 213, 110–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottstein, B.; Deplazes, P. Alveolar echinococcosis: What triggers emergence in North America, Central Europe and Asia? Curr. Opin. Infect. Dis. 2021, 34, 440–446. [Google Scholar] [CrossRef]
- Schweiger, A.; Ammann, R.W.; Candinas, D.; Clavien, P.; Eckert, J.; Gottstein, B.; Halkic, N.; Muellhaupt, B.; Prinz, B.M.; Tarr, P.E.; et al. Human Alveolar Echinococcosis, Switzerland. Emerg. Infect. Dis. 2007, 13, 878–882. [Google Scholar] [CrossRef]
- Budke, C.M.; Carabin, H.; Ndimubanzi, P.C.; Nguyen, H.; Rainwater, E.; Dickey, M.; Bhattarai, R.; Zeziulin, O.; Qian, M.B. A systematic review of the literature on cystic echinococcosis frequency worldwide and its associated clinical manifestations. Am. J. Trop. Med. Hyg. 2013, 88, 1011–1027. [Google Scholar] [CrossRef] [Green Version]
- Piseddu, T.; Brundu, D.; Stegel, G.; Loi, F.; Rolesu, S.; Masu, G.; Ledda, S.; Masala, G. The disease burden of human cystic echinococcosis based on HDRs from 2001 to 2014 in Italy. PLoS Negl. Trop. Dis. 2017, 11, e0005771. [Google Scholar] [CrossRef] [Green Version]
- Tamarozzi, F.; Akhan, O.; Cretu, C.M.; Vutova, K.; Akinci, D.; Chipeva, R.; Ciftci, T.; Constantin, C.M.; Fabiani, M.; Golemanov, B.; et al. Prevalence of abdominal cystic echinococcosis in rural Bulgaria, Romania, and Turkey: A cross-sectional, ultrasound-based, population study from the HERACLES project. Lancet Infect. Dis. 2018, 18, 769–778. [Google Scholar] [CrossRef]
- Craig, P.S. Epidemiology of human alveolar echinococcosis in China. Parasitol. Int. 2006, 55, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Bebezov, B.; Mamashev, N.; Umetaliev, T.; Ziadinov, I.; Craig, P.S.; Joekel, D.E.; Deplazes, P.; Grimm, F.; Torgerson, P.R. Intense focus of alveolar echinococcosis, South Kyrgyzstan. Emerg. Infect. Dis. 2018, 24, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Paternoster, G.; Boo, G.; Wang, C.; Minbaeva, G.; Usubalieva, J.; Raimkulov, K.M.; Zhoroev, A.; Abdykerimov, K.K.; Kronenberg, P.A.; Müllhaupt, B.; et al. Epidemic cystic and alveolar echinococcosis in Kyrgyzstan: An analysis of national surveillance data. Lancet Glob. Health 2020, 8, e603–e611. [Google Scholar] [CrossRef] [Green Version]
- Counotte, M.J.; Minbaeva, G.; Usubalieva, J.; Abdykerimov, K.; Torgerson, P.R. The Burden of Zoonoses in Kyrgyzstan: A Systematic Review. PLoS Negl. Trop. Dis. 2016, 10, e0004831. [Google Scholar] [CrossRef] [Green Version]
- Torgerson, P.R.; Schweiger, A.; Deplazes, P.; Pohar, M.; Ammann, R.W.; Tarr, P.E.; Halkik, N.; Mu, B. Alveolar echinococcosis: From a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J. Hepatol. 2008, 49, 72–77. [Google Scholar] [CrossRef]
- Siles-Lucas, M.; Casulli, A.; Conraths, F.J.; Müller, N. Laboratory Diagnosis of Echinococcus spp. in Human Patients and Infected Animals. Adv. Parasitol. 2017, 96, 159–257. [Google Scholar]
- Manzano-Román, R.; Sánchez-Ovejero, C.; Hernández-González, A.; Casulli, A.; Siles-Lucas, M. Serological Diagnosis and Follow-Up of Human Cystic Echinococcosis: A New Hope for the Future? Biomed Res. Int. 2015, 2015, 428205. [Google Scholar] [CrossRef] [Green Version]
- Schweiger, A.; Grimm, F.; Tanner, I.; Müllhaupt, B.; Bertogg, K.; Müller, N.; Deplazes, P. Serological diagnosis of echinococcosis: The diagnostic potential of native antigens. Infection 2012, 40, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Gottstein, B.; Jacquier, P.; Bresson-Hadni, S.; Eckert, J. Improved primary immunodiagnosis of alveolar echinococcosis in humans by an enzyme-linked immunosorbent assay using the Em2plus antigen. J. Clin. Microbiol. 1993, 31, 373–376. [Google Scholar] [CrossRef] [Green Version]
- Bartholomot, B.; Vuitton, D.A.; Harraga, S.; Shi, D.Z.; Giraudoux, P.; Barnish, G.; Wang, Y.H.; MacPherson, C.N.L.; Craig, P.S. Combined ultrasound and serologic screening for hepatic alveolar Echinococcosis in central China. Am. J. Trop. Med. Hyg. 2002, 66, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Fourth WHO Report on Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2017; ISBN 9789241565448. [Google Scholar]
- Gottstein, B.; Eckert, J.; Fey, H. Serological differentiation between Echinococcus granulosus and E. multilocularis infections in man. Z. Parasitenkd. 1983, 69, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Gottstein, B. A monoclonal antibody against Echinococcus multilocularis Em2 antigen. Parasitology 1991, 103, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hülsmeier, A.J.; Gehrig, P.M.; Geyer, R.; Sack, R.; Gottstein, B.; Deplazes, P.; Köhler, P. A major Echinococcus multilocularis antigen is a mucin-type glycoprotein. J. Biol. Chem. 2002, 277, 5742–5748. [Google Scholar] [CrossRef] [Green Version]
- Auer, H.; Aspöck, H. Serodiagnostik der alveolären Echinokokkose mittels eines Antigens aus in vitro gehaltenen Protoscolices von Echinococcus multilocularis. Mitt. Österr. Ges. Tropenmed. Parasitol. 1989, 11, 13–18. [Google Scholar]
- Gottstein, B.; Lachenmayer, A.; Beldi, G.; Wang, J.; Merkle, B.; Vu, X.L.; Kurath, U.; Müller, N. Diagnostic and follow-up performance of serological tests for different forms/courses of alveolar echinococcosis. Food Waterborne Parasitol. 2019, 16, e00055. [Google Scholar] [CrossRef]
- Müller, N.; Frei, E.; Nunez, S.; Gottstein, B. Improved serodiagnosis of alveolar echinococcosis of humans using an in vitro produced Echinococcus multilocularis antigen. Parasitology 2007, 134, 879–888. [Google Scholar] [CrossRef] [Green Version]
- Sako, Y.; Nakao, M.; Nakaya, K.; Yamasaki, H.; Gottstein, B.; Lightowers, M.W.; Schantz, P.M.; Ito, A. Alveolar Echinococcosis: Characterization of diagnostic antigen Em18 and serological evaluation of recombinant Em18. J. Clin. Microbiol. 2002, 40, 2760–2765. [Google Scholar] [CrossRef] [Green Version]
- Ammann, R.W.; Stumpe, K.D.M.; Grimm, F.; Deplazes, P.; Huber, S.; Bertogg, K.; Fischer, D.R.; Müllhaupt, B. Outcome after Discontinuing Long-Term Benzimidazole Treatment in 11 Patients with Non-resectable Alveolar Echinococcosis with Negative FDG-PET/CT and Anti-EmII/3-10 Serology. PLoS Negl. Trop. Dis. 2015, 9, e0003964. [Google Scholar] [CrossRef]
- Deibel, A.; Stocker, D.; Meyer zu Schwabedissen, C.; Husmann, L.; Kronenberg, P.A.; Grimm, F.; Deplazes, P.; Reiner, C.S.; Müllhaupt, B. Evaluation of a structured treatment discontinuation in patients with inoperable alveolar echinococcosis on long-term benzimidazole therapy: A retrospective cohort study. PLoS Negl. Trop. Dis. 2022, 16, e0010146. [Google Scholar] [CrossRef]
- Hotz, J.F.; Peters, L.; Kapp-Schwörer, S.; Theis, F.; Eberhardt, N.; Essig, A.; Grüner, B.; Hagemann, J.B. Evaluation of Serological Markers in Alveolar Echinococcosis Emphasizing the Correlation of PET-CTI Tracer Uptake with RecEm18 and Echinococcus-Specific IgG. Pathogens 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Lightowlers, M.W.; Jensen, O.; Fernandez, E.; Iriarte, J.A.; Woollard, D.J.; Gauci, C.G.; Jenkins, D.J.; Heath, D.D. Vaccination trials in Australia and Argentina confirm the effectiveness of the EG95 hydatid vaccine in sheep. Int. J. Parasitol. 1999, 29, 531–534. [Google Scholar] [CrossRef]
- Gauci, C.; Merli, M.; Muller, V.; Chow, C.; Yagi, K.; Mackenstedt, U.; Lightowlers, M.W. Molecular cloning of a vaccine antigen against infection with the larval stage of Echinococcus multilocularis. Infect. Immun. 2002, 70, 3969–3972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, C.F.; Marreros, N.; Renneker, S.; Schmidt, L.; Sager, H.; Hentrich, B.; Milesi, S.; Gottstein, B. Dogs as victims of their own worms: Serodiagnosis of canine alveolar echinococcosis. Parasites Vectors 2017, 10, 422. [Google Scholar] [CrossRef]
- Lissandrin, R.; Tamarozzi, F.; Piccoli, L.; Tinelli, C.; De Silvestri, A.; Mariconti, M.; Meroni, V.; Genco, F.; Brunetti, E. Factors influencing the serological response in hepatic Echinococcus granulosus infection. Am. J. Trop. Med. Hyg. 2016, 94, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Hernández-González, A.; Santivañez, S.; García, H.H.; Rodríguez, S.; Muñoz, S.; Ramos, G.; Orduña, A.; Siles-Lucas, M. Improved serodiagnosis of cystic echinococcosis using the new recombinant 2B2t antigen. PLoS Negl. Trop. Dis. 2012, 6, e1714. [Google Scholar] [CrossRef] [Green Version]
- Mubanga, C.; Mwape, K.E.; Phiri, I.K.; Trevisan, C.; Zulu, G.; Chabala, C.; van Damme, I.; Schmidt, V.; Dorny, P.; Gabriël, S. Progress on the development of rapid diagnostic tests for foodborne neglected zoonotic helminthiases: A systematic review. Acta Trop. 2019, 194, 135–147. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Longoni, S.S.; Vola, A.; Degani, M.; Tais, S.; Rizzi, E.; Prato, M.; Scarso, S.; Silva, R.; Brunetti, E.; et al. Evaluation of nine commercial serological tests for the diagnosis of human hepatic cyst echinococcosis and the differential diagnosis with other focal liver lesions: A diagnostic accuracy study. Diagnostics 2021, 11, 167. [Google Scholar] [CrossRef]
- Tappe, D.; Grüner, B.; Kern, P.; Frosch, M. Evaluation of a commercial Echinococcus western blot assay for serological follow-up of patients with alveolar echinococcosis. Clin. Vaccine Immunol. 2008, 15, 1633–1637. [Google Scholar] [CrossRef] [Green Version]
- Deininger, S.; Wellinghausen, N. Evaluation of a new combined Western and line blot assay (EUROLINE-WB) for diagnosis and species identification of Echinococcus infection in humans. GMS Infect. Dis. 2019, 7, Doc01. [Google Scholar]
- Schoenfeld, L.; Scheper, T.; Warnecke, J.M.; Gottstein, B. A Newly Developed Membrane-Based Assay for Simultaneous Serologic Screening and Differentiation of Echinococcoses. 5855591. EUROIMMUN AG, Luebeck, Germany. Available online: https://www.escmid.org/guidelines_publications/escmid_elibrary/material/?mid=48276 (accessed on 23 March 2022).
- Stieger, C.; Hegglin, D.; Mathis, A.; Deplazes, P.; Hegglin, D.; Schwarzenbach, G. Spatial and temporal aspects of urban transmission of Echinococcus multilocularis. Parasitology 2002, 124, 631–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez Rojas, C.A.; Kronenberg, P.A.; Aitbaev, S.; Omorov, R.A.; Abdykerimov, K.K.; Paternoster, G.; Müllhaupt, B.; Torgerson, P.; Deplazes, P. Genetic diversity of Echinococcus multilocularis and Echinococcus granulosus sensu lato in Kyrgyzstan: The A2 haplotype of E. multilocularis is the predominant variant infecting humans. PLoS Negl. Trop. Dis. 2020, 14, e0008242. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, M.; Micheloud, C.; Grimm, F.; Kronenberg, P.A.; Grimm, J.; Beck, A.; Nell, J.; Meyer Zu Schwabedissen, C.; Furrer, E.; Müllhaupt, B.; et al. Pathology of Echinococcosis: A Morphologic and Immunohistochemical Study on 138 Specimens with Focus on the Differential Diagnosis between Cystic and Alveolar Echinococcosis. Am. J. Surg. Pathol. 2020, 44, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Xiao, N.; Okamoto, M.; Yanagida, T.; Sako, Y.; Ito, A. Geographic pattern of genetic variation in the fox tapeworm Echinococcus multilocularis. Parasitol. Int. 2009, 58, 384–389. [Google Scholar] [CrossRef]
- Norman, L.; Kagan, I.G. The Maintenance of Echinococcus multilocularis in Gerbils (Meriones unguiculatus) by Intraperitoneal Inoculation. J. Parasitol. 1961, 47, 870–874. [Google Scholar] [CrossRef]
- Laurimäe, T.; Kronenberg, P.A.; Alvarez Rojas, C.A.; Ramp, T.W.; Eckert, J.; Deplazes, P. Long-term (35 years) cryopreservation of Echinococcus multilocularis metacestodes. Parasitology 2020, 147, 1048–1054. [Google Scholar] [CrossRef]
- Spiliotis, M.; Brehm, K. Axenic In Vitro Cultivation of Echinococcus multilocularis Metacestode Vesicles and the Generationof Primary Cell Cultures. In Host-Pathogen Interactions: Methods and Protocols; Rupp, S., Sohn, K., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 245–262. ISBN 978-1-59745-204-5. [Google Scholar]
- Gauci, C.; Jenkins, D.; Lightowlers, M.W. Strategies for optimal expression of vaccine antigens from taeniid cestode parasites in escherichia coli. Mol. Biotechnol. 2011, 48, 277–289. [Google Scholar] [CrossRef]
- Fu, C.; Donovan, W.P.; Shikapwashya-Hasser, O.; Ye, X.; Cole, R.H. Hot fusion: An efficient method to clone multiple DNA fragments as well as inverted repeats without ligase. PLoS ONE 2014, 9, e115318. [Google Scholar] [CrossRef]
- Lorenzo, C.; Ferreira, H.B.; Monteiro, K.M.; Rosenzvit, M.; Kamenetzky, L.; García, H.H.; Vasquez, Y.; Naquira, C.; Sánchez, E.; Lorca, M.; et al. Comparative analysis of the diagnostic performance of six major Echinococcus granulosus antigens assessed in a double-blind, randomized multicenter study. J. Clin. Microbiol. 2005, 43, 2764–2770. [Google Scholar] [CrossRef] [Green Version]
- Steinmann, P.; Usubalieva, J.; Imanalieva, C.; Minbaeva, G.; Stefiuk, K.; Jeandron, A.; Utzinger, J. Rapid appraisal of human intestinal helminth infections among schoolchildren in osh oblast, kyrgyzstan. Acta Trop. 2010, 116, 178–184. [Google Scholar] [CrossRef]
- Craig, P.S.; Zeyhle, E.; Romig, T. Hydatid disease: Research and control in turkana. II. the role of immunological techniques for the diagnosis of hydatid disease. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 183–192. [Google Scholar] [CrossRef]
- Mohammadzadeh, T.; Sako, Y.; Sadjjadi, S.M.; Sarkari, B.; Ito, A. Comparison of the usefulness of hydatid cyst fluid, native antigen B and recombinant antigen B8/1 for serological diagnosis of cystic echinococcosis. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 371–375. [Google Scholar] [CrossRef] [PubMed]
| Parasitic Diseases (N = 144) | Entamoeba | histolytica | Taenia | solium | Ascaris | lumbricoides | Trichinella | spp. | Strongyloides | stercoralis | Toxocara | spp. | Shistosoma | spp. | Fasciola | hepatica | Filarial | Species | Total | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | CH | KG | CH | KG | CH | KG | CH | KG | CH | KG | CH | KG | CH | KG | CH | KG | CH | KG | CH | KG |
| Number of sera | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 144 | N = 144 |
| Antigens for ELISA | ||||||||||||||||||||
| EmVF | 0 | 0 | 3 | 2 | 4 | 2 | 7 | 5 | 3 | 2 | 1 | 0 | 0 | 0 | 2 | 0 | 8 | 7 | 19.4% | 12.5% |
| EmVC | 0 | 0 | 4 | 3 | 4 | 3 | 4 | 4 | 1 | 0 | 3 | 0 | 2 | 0 | 4 | 4 | 5 | 4 | 18.8% | 12.5% |
| EmP | 0 | 0 | 3 | 3 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 2 | 4 | 6.3% | 8.3% |
| Em2G11 | 0 | 0 | 1 | 1 | 5 | 4 | 6 | 3 | 4 | 2 | 2 | 1 | 7 | 3 | 7 | 6 | 9 | 9 | 28.5% | 20.1% |
| mAb EmG3-EmVC | 0 | 0 | 2 | 2 | 6 | 7 | 4 | 4 | 1 | 2 | 1 | 1 | 0 | 0 | 2 | 3 | 8 | 8 | 16.7% | 18.8% |
| recEm18 | 0 | 0 | 0 | 0 | 4 | 4 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 5.6% | 5.6% |
| recEm95 | 2 | 5 | 5 | 9 | 3 | 7 | 8 | 11 | 6 | 12 | 8 | 10 | 5 | 7 | 3 | 9 | 2 | 5 | 29.2% | 52.1% |
| mAb EmG3-EgVC | 0 | 0 | 4 | 4 | 5 | 5 | 7 | 7 | 1 | 1 | 1 | 1 | 0 | 0 | 7 | 7 | 7 | 7 | 22.2% | 22.2% |
| EgVC | 1 | 1 | 4 | 4 | 2 | 2 | 7 | 7 | 0 | 0 | 2 | 2 | 0 | 0 | 7 | 7 | 4 | 4 | 18.8% | 18.8% |
| EgCF | 0 | 0 | 8 | 7 | 9 | 8 | 7 | 5 | 9 | 2 | 0 | 0 | 0 | 0 | 7 | 5 | 15 | 15 | 38.2% | 29.2% |
| EgP | 0 | 0 | 8 | 7 | 4 | 3 | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 5 | 4 | 10 | 8 | 22.2% | 17.4% |
| EgAgB | 0 | 0 | 4 | 4 | 8 | 9 | 7 | 7 | 8 | 10 | 0 | 0 | 0 | 0 | 9 | 11 | 15 | 15 | 35.4% | 38.9% |
| recEg2B2 | 2 | 2 | 6 | 6 | 9 | 9 | 4 | 4 | 5 | 5 | 9 | 9 | 12 | 12 | 4 | 4 | 9 | 9 | 41.7% | 41.7% |
| Commercial test | ||||||||||||||||||||
| Western blot-species | 13 | 13 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11.1% | 11.1% |
| Western blot-genus | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 5 | 5 | 9.7% | 9.7% |
| EmVF-ELISA | 0 | 0 | 0 | 0 | 6 | 6 | 5 | 5 | 2 | 2 | 0 | 0 | 0 | 0 | 5 | 5 | 8 | 8 | 18.1% | 18.1% |
| EgP-IFAT | 0 | 0 | 7 | 7 | 8 | 8 | 3 | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 9 | 9 | 10 | 10 | 27.8% | 27.8% |
| VectorBest-ELISA | 0 | 0 | 1 | 1 | 3 | 3 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 12 | 12 | 16.0% | 16.0% |
| Count of positive tests | 19 | 22 | 62 | 62 | 86 | 87 | 81 | 76 | 44 | 41 | 27 | 24 | 26 | 22 | 78 | 81 | 132 | 133 |
| Parasitic Diseases (N = 144) | Entamoeba | histolytica | Taenia | solium | Ascaris | lumbricoides | Trichinella | spp. | Strongyloides | stercoralis | Toxocara | spp. | Shistosoma | spp. | Fasciola | hepatica | Filarial | Species | Total | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | CH | KG | CH | KG | CH | KG | CH | KG | CH | KG | CH | KG | CH | KG | CH | KG | CH | KG | CH | KG |
| Number of sera | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 16 | N = 144 | N = 144 |
| Antigens for ELISA | ||||||||||||||||||||
| EmVF | 0 | 0 | 4 | 1 | 10 | 4 | 7 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 14 | 10 | 34.0% | 13.9% |
| EmVC | 5 | 2 | 6 | 5 | 7 | 6 | 6 | 5 | 6 | 5 | 3 | 3 | 4 | 2 | 7 | 6 | 7 | 6 | 35.4% | 27.8% |
| EmP | 3 | 3 | 6 | 6 | 5 | 5 | 2 | 2 | 10 | 10 | 2 | 2 | 3 | 3 | 3 | 3 | 4 | 4 | 26.4% | 26.4% |
| mAb EmG3-EmVC | 0 | 0 | 2 | 4 | 7 | 9 | 7 | 8 | 4 | 4 | 1 | 3 | 0 | 0 | 4 | 5 | 8 | 9 | 22.9% | 29.1% |
| mAb EmG3-EgVC | 0 | 0 | 6 | 5 | 7 | 7 | 8 | 8 | 3 | 2 | 2 | 1 | 2 | 0 | 8 | 7 | 9 | 9 | 31.3% | 27.1% |
| EgVC | 5 | 1 | 5 | 2 | 5 | 2 | 9 | 5 | 1 | 0 | 8 | 1 | 1 | 0 | 8 | 4 | 5 | 2 | 32.6% | 11.8% |
| EgCF | 0 | 0 | 7 | 7 | 8 | 8 | 9 | 5 | 11 | 2 | 1 | 0 | 0 | 0 | 11 | 5 | 15 | 15 | 43.1% | 29.2% |
| EgP | 0 | 0 | 8 | 7 | 7 | 3 | 7 | 3 | 4 | 0 | 0 | 0 | 0 | 0 | 8 | 4 | 12 | 8 | 31.9% | 17.4% |
| EgAgB | 0 | 0 | 4 | 4 | 9 | 8 | 7 | 6 | 11 | 8 | 0 | 0 | 0 | 0 | 11 | 8 | 15 | 15 | 39.6% | 34.0% |
| recEg2B2 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 2 | 2 | 5 | 5 | 8.3% | 8.3% |
| Commercial test | ||||||||||||||||||||
| Western blot-species | 13 | 13 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11.1% | 11.1% |
| Western blot-genus | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 5 | 5 | 9.7% | 9.7% |
| EmVF-ELISA | 0 | 0 | 0 | 0 | 6 | 6 | 5 | 5 | 2 | 2 | 0 | 0 | 0 | 0 | 5 | 5 | 8 | 8 | 18.1% | 18.1% |
| EgP-IFAT | 0 | 0 | 7 | 7 | 8 | 8 | 3 | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 9 | 9 | 10 | 10 | 27.8% | 27.8% |
| VectorBest-ELISA | 0 | 0 | 1 | 1 | 3 | 3 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 12 | 12 | 16.0% | 16.0% |
| Count of positive tests | 27 | 20 | 60 | 53 | 87 | 74 | 77 | 62 | 60 | 36 | 18 | 11 | 12 | 7 | 89 | 62 | 129 | 118 |
| Test-Combinations | Sensitivity | Specificity | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| (ELISA) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| Switzerland | Switzerland | Kyrgyzstan | Kyrgyzstan | ||
| AE * patients | blood donors | AE * patients | Ultrasound negative patients | ||
| Antigen 1 | Antigen 2 | N = 60 | N = 68 | N = 40 | N = 68 |
| mAb EmG3-EmVC | EmVF | 0.97 (0.88–0.99) | 0.99 (0.92–0.99) | 0.70 (0.53–0.83) | 0.90 (0.80–0.96) |
| mAb EmG3-EmVC | EmVC | 0.97 (0.88–0.99) | 0.99 (0.92–0.99) | 0.68 (0.51–0.81) | 0.85 (0.75–0.93) |
| mAb EmG3-EmVC | EmP | 0.97 (0.88–0.99) | 0.94 (0.86–0.98) | 0.68 (0.51–0.81) | 0.88 (0.78–0.95) |
| mAb EmG3-EmVC | Em2G11 | 0.97 (0.88–0.99) | 0.97 (0.90–0.99) | 0.75 (0.59–0.87) | 0.83 (0.72–0.92) |
| mAb EmG3-EmVC | recEm18 | 0.97 (0.88–0.99) | 0.94 (0.86–0.98) | 0.66 (0.51–0.81) | 0.81 (0.70–0.89) |
| mAb EmG3-EmVC | recEm95 | 0.97 (0.88–0.99) | 0.76 (0.64–0.86) | 0.98 (0.87–0.99) | 0.85 (0.75–0.93) |
| mAb EmG3-EmVC | EgP | 0.97 (0.90–0.99) | 0.97 (0.90–0.99) | 0.68 (0.51–0.81) | 0.90 (0.73–0.92) |
| mAb EmG3-EmVC | EgCF | 0.99 (0.92–0.99) | 0.99 (0.92–0.99) | 0.75 (0.59–0.87) | 0.87 (0.76–0.94) |
| EmVF | EmVC | 0.95 (0.86–0.99) | 1.00 (0.94–1.00) | 0.65 (0.48–0.79) | 0.90 (0.80–0.96) |
| EmVF | EmP | 0.95 (0.86–0.99) | 0.96 (0.87–0.99) | 0.73 (0.56–0.85) | 0.90 (0.80–0.96) |
| EmVF | Em2G11 | 0.92 (0.82–0.97) | 0.99 (0.92–0.99) | 0.73 (0.56–0.85) | 0.87 (0.76–0.94) |
| EmVF | recEm18 | 0.92 (0.82–0.97) | 0.96 (0.87–0.99) | 0.65 (0.48–0.79) | 0.85 (0.75–0.93) |
| EmVF | recEm95 | 0.92 (0.82–0.97) | 0.77 (0.66–0.87) | 0.93 (0.80–0.98) | 0.90 (0.80–0.96) |
| EmVF | EgP | 0.92 (0.82–0.97) | 0.99 (0.92–0.99) | 0.68 (0.51–0.81) | 0.91 (0.82–0.97) |
| EmVF | EgCF | 1.00 (0.94–1.00) | 1.00 (0.95–1.00) | 0.70 (0.53–0.83) | 0.88 (0.78–0.95) |
| EmP | EmVC | 0.95 (0.86–0.99) | 0.96 (0.87–0.99) | 0.70 (0.53–0.83) | 0.87 (0.76–0.94) |
| EmP | Em2G11 | 0.97 (0.88–0.99) | 0.94 (0.86–0.98) | 0.76 (0.61–0.89) | 0.82 (0.71–0.91) |
| EmP | recEm18 | 0.97 (0.88–0.99) | 0.91 (0.81–0.97) | 0.70 (0.53–0.83) | 0.82 (0.71–0.91) |
| EmP | recEm95 | 0.97 (0.88–0.99) | 0.75 (0.63–0.85) | 0.98 (0.87–0.99) | 0.87 (0.76–0.94) |
| EmP | EgP | 0.97 (0.88–0.99) | 0.96 (0.88–0.99) | 0.68 (0.51–0.81) | 0.87 (0.76–0.94) |
| EmP | EgCF | 0.97 (0.88–0.99) | 0.96 (0.88–0.99) | 0.78 (0.62–0.89) | 0.84 (0.73–0.92) |
| Em2G11 | EmVC | 0.97 (0.88–0.99) | 0.99 (0.92–0.99) | 0.73 (0.56–0.85) | 0.82 (0.71–0.91) |
| Em2G11 | recEm18 | 0.92 (0.81–0.97) | 0.94 (0.86–0.98) | 0.73 (0.56–0.85) | 0.76 (0.65–0.86) |
| Em2G11 | recEm95 | 0.93 (0.84–0.98) | 0.78 (0.66–0.87) | 0.98 (0.87–0.99) | 0.81 (0.70–0.89) |
| Em2G11 | EgP | 0.92 (0.82–0.97) | 0.97 (0.90–0.99) | 0.75 (0.59–0.87) | 0.84 (0.73–0.92) |
| Em2G11 | EgCF | 0.92 (0.82–0.97) | 0.99 (0.92–0.99) | 0.73 (0.56–0.85) | 0.84 (0.73–0.92) |
| recEm18 | EmVC | 0.97 (0.88–0.99) | 0.96 (0.88–0.99) | 0.65 (0.48–0.79) | 0.82 (0.71–0.91) |
| recEm18 | recEm95 | 0.90 (0.79–0.96) | 0.76 (0.64–0.86) | 0.90 (0.76–0.97) | 0.85 (0.75–0.93) |
| recEm18 | EgP | 0.92 (0.82–0.97) | 0.94 (0.86–0.98) | 0.65 (0.48–0.79) | 0.84 (0.73–0.92) |
| recEm18 | EgCF | 0.92 (0.82–0.97) | 0.96 (0.88–0.99) | 0.68 (0.51–0.81) | 0.79 (0.68–0.88) |
| recEm95 | EmVC | 0.97 (0.88–0.99) | 0.77 (0.66–0.87) | 0.95 (0.83–0.99) | 0.85 (0.75–0.93) |
| recEm95 | EgP | 0.93 (0.84–0.98) | 0.76 (0.65–0.86) | 0.95 (0.83–0.99) | 0.87 (0.76–0.94) |
| recEm95 | EgCF | 0.93 (0.84–0.98) | 0.78 (0.66–0.87) | 0.93 (0.80–0.98) | 0.82 (0.71–0.91) |
| EmVC | EgP | 0.97 (0.88–0.99) | 0.99 (0.92–0.99) | 0.65 (0.34–0.79) | 0.88 (0.78–0.95) |
| EmVC | EgCF | 0.97 (0.88–0.99) | 1.00 (0.95–1.00) | 0.70 (0.53–0.83) | 0.84 (0.73–0.92) |
| EgP | EgCF | 0.92 (0.82–0.97) | 0.99 (0.92–0.99) | 0.73 (0.56–0.85) | 0.88 (0.78–0.95) |
| Test-Combinations | Sensitivity | Specificity | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| (ELISA) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| Switzerland | Switzerland | Kyrgyzstan | Kyrgyzstan | ||
| CE * patients | blood donors | CE * patients | Ultrasound negative patients | ||
| Antigen 1 | Antigen 2 | N = 41 | N = 68 | N = 23 | N = 68 |
| mAb EmG3-EgVC | EgCF | 0.90 (0.77–0.97) | 0.94 (0.86–0.98) | 0.65 (0.43–0.84) | 0.84 (0.73–0.92) |
| mAb EmG3-EgVC | EgP | 0.93 (0.80–0.98) | 0.94 (0.86–0.98) | 0.74 (0.52–0.90) | 0.85 (0.75–0.93) |
| mAb EmG3-EgVC | AgB | 0.93 (0.80–0.98) | 0.93 (0.84–0.98) | 0.74 (0.52–0.90) | 0.75 (0.63–0.85) |
| mAb EmG3-EgVC | recEg2B2 | 0.78 (0.62–0.89) | 0.88 (0.78–0.95) | 0.83 (0.61–0.95) | 0.85 (0.75–0.93) |
| mAb EmG3-EgVC | EgVC | 0.75 (0.60–0.88) | 0.81 (0.70–0.89) | 0.59 (0.36–0.79) | 0.63 (0.51–0.75) |
| EgP | EgCF | 0.90 (0.77–0.97) | 1.00 (0.95–1.00) | 0.74 (0.52–0.90) | 0.87 (0.76–0.94) |
| EgP | AgB | 0.93 (0.80–0.98) | 0.99 (0.92–0.99) | 0.78 (0.56–0.93) | 0.78 (0.66–0.87) |
| EgP | recEg2B2 | 0.93 (0.80–0.98) | 0.93 (0.84–0.98) | 0.87 (0.66–0.97) | 0.89 (0.80–0.96) |
| EgP | EgVC | 0.93 (0.80–0.98) | 0.84 (0.73–0.92) | 0.74 (0.52–0.90) | 0.63 (0.51–0.75) |
| AgB | EgCF | 0.90 (0.77–0.97) | 0.99 (0.92–0.99) | 0.70 (0.47–0.87) | 0.76 (0.65–0.86) |
| AgB | recEg2B2 | 0.93 (0.80–0.98) | 0.91 (0.82–0.97) | 0.83 (0.61–0.95) | 0.79 (0.68–0.88) |
| AgB | EgVC | 0.90 (0.77–0.97) | 0.82 (0.71–0.91) | 0.74 (0.52–0.90) | 0.59 (0.46–0.71) |
| recEg2B2 | EgCF | 0.90 (0.77–0.97) | 0.93 (0.84–0.98) | 0.78 (0.56–0.93) | 0.83 (0.73–0.92) |
| recEg2B2 | EgVC | 0.78 (0.62–0.89) | 0.63 (0.51–0.75) | 0.83 (0.61–0.95) | 0.66 (0.54–0.77) |
| EgVC | EgCF | 0.88 (0.73–0.96) | 0.84 (0.73–0.92) | 0.68 (0.45–0.86) | 0.62 (0.49–0.73) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kronenberg, P.A.; Deibel, A.; Gottstein, B.; Grimm, F.; Müllhaupt, B.; Meyer zu Schwabedissen, C.; Aitbaev, S.; Omorov, R.A.; Abdykerimov, K.K.; Minbaeva, G.; et al. Serological Assays for Alveolar and Cystic Echinococcosis—A Comparative Multi-Test Study in Switzerland and Kyrgyzstan. Pathogens 2022, 11, 518. https://doi.org/10.3390/pathogens11050518
Kronenberg PA, Deibel A, Gottstein B, Grimm F, Müllhaupt B, Meyer zu Schwabedissen C, Aitbaev S, Omorov RA, Abdykerimov KK, Minbaeva G, et al. Serological Assays for Alveolar and Cystic Echinococcosis—A Comparative Multi-Test Study in Switzerland and Kyrgyzstan. Pathogens. 2022; 11(5):518. https://doi.org/10.3390/pathogens11050518
Chicago/Turabian StyleKronenberg, Philipp A., Ansgar Deibel, Bruno Gottstein, Felix Grimm, Beat Müllhaupt, Cordula Meyer zu Schwabedissen, Sezdbek Aitbaev, Rakhatbek A. Omorov, Kubanychbek K. Abdykerimov, Gulnara Minbaeva, and et al. 2022. "Serological Assays for Alveolar and Cystic Echinococcosis—A Comparative Multi-Test Study in Switzerland and Kyrgyzstan" Pathogens 11, no. 5: 518. https://doi.org/10.3390/pathogens11050518