Occurrence and Distribution of Fusarium Communities in the Root Zone in a Post-Bog Permanent Meadow in Relation to Mineral Fertilization and Growing Seasons
Abstract
:1. Introduction
2. Results
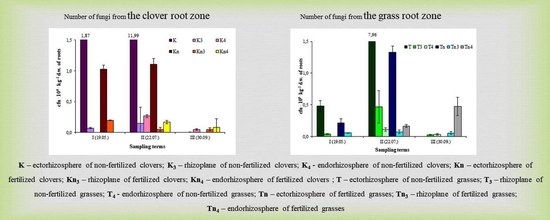
2.1. Numbers of Fungi Isolated on the Nash and Snyder Medium
2.2. Fusarium and Genera Related to Fusarium
2.3. Distribution of the Fusarium and Genera Related to Fusarium in the Root Zone
2.3.1. Ectorhizosphere and Endorhizosphere of Clovers
2.3.2. Rhizoplane of Clovers
2.3.3. Ectorhizosphere and Endorhizosphere of Grasses
2.3.4. Rhizoplane of Grasses
2.4. Similarity and Species Diversity of Fusarium and Genera Related to Fusarium in Root Zone of Clover–Grass Sward
2.4.1. Clover Root Zone
2.4.2. Grass Root Zone
2.5. Relationships between Frequency of Isolation of Fungi and Colonized Root Zone Microenvironment
3. Discussion
3.1. Abundance of Fusarium in Plant Root Zone
3.2. Species Richness, Similarity, and Diversity in Fusarium Communities Colonizing the Plant Root Zone
4. Materials and Methods
4.1. Study Area
4.2. Isolation and Identification of Fungi
4.3. Analysis of Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipińska, H. Assessment of the persistence of Poa pratensis L., Phleum pratense L., and Lolium pratense L. in sward on peat-muck soil depending on the groundwater level. In Scientific Research Series of the University of Life Sciences in Lublin, 347; University of Life Sciences in Lublin Publishing House: Lublin, Poland, 2010. (In Polish) [Google Scholar]
- Dąbek-Szreniawska, M. Microbiological characteristics of peat-muck soils subjected to drainage and wetting. Acta Agrophys. 2002, 68, 21–28. (In Polish) [Google Scholar]
- Gotkiewicz, G.; Kowalczyk, Z. Differentiation of biological processes in basic types of soils in post-bog habitats. Adv. Agric. Sci. Probl. Issues 1977, 186, 97–118. (In Polish) [Google Scholar]
- Wyczółkowski, A.; Bieganowski, A.; Malicki, J. Determination of the abundance of microorganisms in peat-muck soil with various degrees of mucking. Ann. UMCS Sec. E 2002, 57, 93–98. (In Polish) [Google Scholar]
- Tyszkiewicz, Z. Fungal communities of three weakly mucked peat-muck soils. Acta Agrobot. 2003, 55, 335–345. (In Polish) [Google Scholar] [CrossRef]
- Tyszkiewicz, Z. Fungi in selected peat and peat-muck soils. In Nature of Podlasie. Narew National Park. Nature Monograph; Banaszuk, H., Ed.; Economics and Environment Publishing House: Bialystok, Poland, 2004; pp. 185–192. (In Polish) [Google Scholar]
- Tyszkiewicz, Z. Diversity of fungal communities in strongly mucked peat-muck soil. Environ. Prot. Nat. Resour. 2008, 35/36, 149–152. (In Polish) [Google Scholar]
- Tyszkiewicz, Z. Fungi of selected weakly mucked peat-muck soils in the northern part of the Narew National Park. Water-Environ. -Rural. Areas 2010, 10, 211–218. (In Polish) [Google Scholar]
- Korniłłowicz-Kowalska, T.; Wojdyło-Kotwica, B.; Kwiatkowska, E. Changes in the spore number of fungi and in AM colonization of root of clovers and grasses on a peat-muck soil with respect to mineral fertilization. Pak. J. Bot. 2016, 48, 729–798. [Google Scholar]
- Korniłłowicz-Kowalska, T.; Wojdyło-Kotwica, B.; Bohacz, J.; Możejko, M.T. Biodiversity of saprotrophic fungi of the root zone of grasses and clovers in a permanent meadow in a post-bog habitat in relation to the growing season and fertilization (Zaklęsłość Sosnowicka, Western Polesye). In Environmental Engineering in Polesye, Book 3 Polish Polesye; Urban, D., Dobrowolski, R., Jeznach, J., Eds.; International Scientific Publications, Brest-Rivne-Warsaw-Ryazan, Belarus-Ukraine-Poland-Russia; Warsaw University of Life Sciences Publishing House: Warsaw, Poland, 2020; pp. 485–538. (In Polish) [Google Scholar]
- Wojdyło-Kotwica, B.; Korniłłowicz-Kowalska, T. Fungi in the rhizosphere of clovers and grasses. In Environmental Aspects of Plant and Animal Production; Kowalczyk, K., Ed.; University of Life Sciences in Lublin Publishing House: Lublin, Poland, 2012; pp. 53–67. (In Polish) [Google Scholar]
- Wilberforce, E.M.; Boddy, L.; Griffiths, R.; Griffith, G.W.; Wilberforce, E.M.; Boddy, L.; Griffiths, R.; Griffith, G.W. Agricultural management affects communities of culturable root-endophytic fungi in temperate grassland. Soil Biol. Biochem. 2003, 35, 1143–1154. [Google Scholar] [CrossRef]
- Dorenda, M. Research on the microflora of the environment of red clover and cat grass cultivation in the phytopathological aspect. Acta Mycol. 1986, 22, 15–34. (In Polish) [Google Scholar] [CrossRef]
- Korniłłowicz, T. Effect of intensive fertilization with manure and keratin-bark-urea granulate on selected soil mycoflora communities. Adv. Agric. Sci. Probl. Issues 1989, 370, 85–96. (In Polish) [Google Scholar]
- Korniłłowicz-Kowalska, T.; Iglik, H.; Wojdyło, B. Correlation between the abundance of cellulolytic fungi and selected soil properties. Acta Mycol. 2003, 38, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Kutrzeba, M. Soil mycoflora as a factor limiting the occurrence of fungi pathogenic for three varieties of Dactylis glomerata L. Acta Mycol. 1983, 19, 245–281. (In Polish) [Google Scholar] [CrossRef] [Green Version]
- McMullen, M.P.; Stock, R.W. Fusarium species associated with grassland soils. Can. J. Bot. 1983, 61, 2530–2538. [Google Scholar] [CrossRef]
- Peterson, E.A. Observations on fungi associated with plant roots. Can. J. Microbiol. 1958, 4, 257–265. [Google Scholar] [CrossRef]
- Mangla, S.; Callaway, R.M. Exotic invasive plant accumulates native soil pathogens which inhabit native plants. J. Ecol. 2008, 96, 58–67. [Google Scholar] [CrossRef]
- Alabouvette, C. Biological control of Fusarium wilts. In Environmental Biotic Factors in Integrated Plant Disease Control; Marika, M., Ed.; The Polish Mytopathol Society: Poznań, Poland, 1995; pp. 61–65. [Google Scholar]
- Murase, J.; Shibata, M.; Lee, C.G.; Watanabe, T.; Asakawa, S.; Kimura, M. Incorporation of plant residue-derived carbon into the microeukaryotic community in a rice field soil revealed by DNA stable-isotope probing. FEMS Microbiol. Ecol. 2012, 79, 371–379. [Google Scholar] [CrossRef]
- Nash, S.M.; Snyder, W.C. Quantitative estimations by plate counts of propagules of the Bean root rot Fusarium in field soils. Phytopathology 1962, 52, 567–572. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil FUNGI, 2nd ed.; IHW-Verlag: Eching, Germany, 2007. [Google Scholar]
- Bills, G.F.; Christensen, M.; Pawell, M.; Horn, G. Saprobic soil fungi. In Biodiversity of Fungi Inventory and Monitoring Methods; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; pp. 271–302. [Google Scholar]
- Nur Ain Izzati, M.Z.; Siti Nordahliawate, M.S.; Nor Azliza, I.; Sallen, B. Distribution and diversity of Fusarium species associated with grasses in ten states throughout Peninsular Malaysia. Biotropia 2009, 16, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Kovačikova, E.; Kůdela, V. Fusarium species associated with root rot of red clover. Zbl. Mikrobiol. 1982, 137, 407–414. [Google Scholar] [CrossRef]
- LeBlanc, N.; Kinkel, L.L.; Kistler, H.C. Soil fungal communities respond to grassland plant community richness and soil edaphics. Microb. Ecol. 2015, 70, 188–195. [Google Scholar] [CrossRef]
- Shivanna, M.B.; Vasanthakumari, M.M. Temporal and spatial variability of rhizosphere and rhizoplane fungal communities in grasses of the subfamily Chloridoideae in the Lakkavalli region of the Western Ghats in India. Mycosphere 2011, 2, 255–271. [Google Scholar]
- Vasanthakumari, M.M.; Shivanna, M.B. Fungal assemblages in rhizosphere and rhizoplane of the grasses of the subfamily Panicoideae in the Lakkavalli region of Karnataka, India. Microbes Environ. 2011, 26, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Yli-Mattila, T.; Kalko, G.; Hannukkala, A.; Paavanen-Huhtala, S.; Hakala, K. Prevalence, species, composition, genetic variation and pathogenicity of clover rot (Sclerotinia trifoliorum) and Fusarium spp. in red clover in Finland. Eur. J. Plant Pathol. 2010, 126, 13–27. [Google Scholar] [CrossRef]
- Kurek, E.; Machowicz, Z.; Kulpa, D.; Słomka, A. The microorganisms of rye (Secale cereale L.) rhizosphere. Acta Microbiol. Pol. 1994, 43, 251–255. [Google Scholar]
- Wichern, F.; Eberhardt, E.; Mayer, J.; Joergensen, R.G.; Müller, T. Nitrogen rhizodeposition in agricultural crops: Methods, estimates and future prospects. Soil Biol. Biochem. 2008, 40, 30–48. [Google Scholar] [CrossRef]
- Strzelczyk, E. Biological control of soil pathogens in plants. Adv. Microbiol. 1988, 27, 255–272. (In Polish) [Google Scholar]
- Dorenda, M. The formation of fungal associations in a mountain agricultural habitat of Trifolium pratense L. and Dactylis glomerata L. Acta Mycol 1982, 18, 243–280. (In Polish) [Google Scholar] [CrossRef]
- Grant, C.; Hunter, C.A.; Flannigan, B.; Bravary, A.F. The moisture requirements of moulds isolated from domestic dwellings. Int. Biodeter. Biodegr. 1989, 25, 259–284. [Google Scholar] [CrossRef]
- Griffin, D.M. Ecology of Soil Fungi; Chapman and Hall: London, UK, 1972. [Google Scholar]
- Broeckling, C.D.; Broz, A.K.; Bergelson, J.; Manter, D.K.; Vivanco, J.M. Root exudates regulate soil fungi community composition and diversity. Appl. Env. Microbiol. 2008, 74, 738–744. [Google Scholar] [CrossRef] [Green Version]
- De Vries, F.T.; Hoffland, E.; van Eekeren, N.; Brussaard, L.; Bloem, J. Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol. Biochem. 2006, 38, 2092–2103. [Google Scholar] [CrossRef] [Green Version]
- Twining, J.R.; Zaw, M.; Russell, R.; Wilde, K. Seasonal changes of redox potential and microbial activity in two agricultural soils of tropical Australia: Some implications for soil-to-plant transfer of radionuclides. J. Environ. Radioact. 2004, 76, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Bissett, J.; Parkinson, D. Functional relationships between soil fungi and environment in alpine tundra. Can. J. Bot. 1979, 57, 1642–1659. [Google Scholar] [CrossRef]
- Lynch, J.M.; Whipps, J.M. Substrate flow in the rhizosphere. Plant Soil. 1990, 129, 1–10. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, C.; Lin, F.; Kubicek, C.P. Identity, diversity and molecular phylogeny of the endophytic mycobiota in the roots of rare wild rice (Oryza granulate) from a nature reserve in Yunnan, China. Appl. Environ. Microbiol. 2010, 76, 1642–1652. [Google Scholar] [CrossRef] [Green Version]
- Hoyos-Carvajal, L.; Orduz, S.; Bissett, J. Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol. Control 2009, 51, 409–416. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T. Effect of soil fungi (Micromycetes) on pathogens and plant pests and its practical aspect. Fragm. Agron. 2000, 2, 135–155. (In Polish) [Google Scholar]
- LeBlanc, N.; Kinkel, L.L.; Kistler, H.C. Plant diversity and plant identity influence Fusarium communities in soil. Mycologia 2017, 109, 128–139. [Google Scholar] [CrossRef]
- Korniłłowicz, T. Studies on mycoflora colonizing raw keratin wastes in arable soil. Acta Mycol. 1992, 27, 231–245. (In Polish) [Google Scholar] [CrossRef] [Green Version]
- Korniłłowicz-Kowalska, T.; Bohacz, J. Some correlations between the occurrence frequency of keratinophilic fungi and selected soil properties. Acta Mycol. 2002, 27, 101–116. [Google Scholar] [CrossRef] [Green Version]
- Harrow, S.A.; Farrokhi-Nejad, R.; Pitman, A.R.; Scott, I.A.W.; Bentley, A.; Hide, C.; Cromey, M.G. Characterization of New Zealand Fusarium populations using a polyphasis approach differentiates the F. avenaceum/F. acuminatum/F. tricinctum species complex in cereal and grassland systems. Fungal Biol. 2010, 114, 293–311. [Google Scholar] [CrossRef]
- Kommedahl, T.; Windels, C.E.; Lang, D.S. Comparison of Fusarium populations in grassland of Minnesota and Iceland. Mycologia 1975, 67, 38–44. [Google Scholar] [CrossRef]
- Dorenda, M. Microflora as a factor limiting the presence of pathogenic fungi in red clover crops in pure cultivation with cat grass. In Agricultural University in Wroclaw Scientific Issues. Serial Dissertations; Agricultural Academy Publishing House: Wroclaw, Poland, 1985; pp. 5–26. (In Polish) [Google Scholar]
- Olivain, C.; Trouvelot, S.; Binet, M.N.; Cordier, C.; Pugin, A.; Alabouvette, C. Colonization of flax roots and early physiological responses of flax cells inoculated with pathogenic and nonpathogenic strains of Fusarium oxysporum. Appl. Environ. Microb. 2003, 69, 5453–5462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemanceau, P.; Alabouvette, C. Biological control of Fusarium diseases by fluorescent Pseudomonas and nonpathogenic Fusarium. Crop Prot. 1991, 10, 279–286. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Kurek, E.; Winiarczyk, K.; Baturo, A.; Łukanowski, A. Colonization of root tissues and protection against Fusarium wilt of rye (Secale cereale) by nonpathogenic rhizosphere strains of Fusarium culmorum. Biol. Control 2008, 45, 297–307. [Google Scholar] [CrossRef]
- Kiecana, I.; Cegiełko, M.; Mielniczuk, E.; Pastucha, A. Fungi affecting ornamental grasses and the pathogenicity of Fusarium culmorum (W.G.Sm.) Sacc. and Fusarium equiseti (Corda) Sacc. to selected species. Acta Sci. Pol. Hortorum. 2014, 13, 61–75. [Google Scholar]
- Kurek, E.; Jaroszuk, J. Occurrence of beneficial and deleterious for plant growth Fusarium strains in rye (Secale cereale L.) rhizosphere. Acta Microbiol. Pol. 1994, 43, 181–187. [Google Scholar]
- Kwaśna, H.; Chełkowski, J.; Zajkowski, P. Polish Flora. Fungi (Mycota) v.22 (Deuteromycetes), (Hyphomycetales), (Fusarium); PWN: Warsaw-Krakow, Poland, 1991. (In Polish) [Google Scholar]
- Turner, A.S.; Lees, A.K.; Rezanoor, H.N.; Nicholson, P. Refinement of PCR-detection of Fusarium avenaceum and evidence from DNA marker studies for phenetic relatedness to Fusarium tricinctum. Plant Pathol. 1998, 47, 278–288. [Google Scholar] [CrossRef]
- Olivares, B.O.; Araya-Alman, M.; Acevedo-Opazo, C.; Rey, J.C.; Cañete-Salinas, P.; Kurina, F.G.; Balzarini, M.; Lobo, D.; Navas-Cortés, J.A.; Landa, B.B.; et al. Relationship Between Soil Properties and Banana Productivity in the Two Main Cultivation Areas in Venezuela. J. Soil Sci. Plant Nutr. 2020, 20, 2512–2524. [Google Scholar] [CrossRef]
- Soto, J.; Diaz, J.; Ramírez, M. Diagnostics floristic and phytosanitary of species tree present in the parish Francisco Eugenio Bustamante, Maracaibo, Zulia state, Venezuela. Rev. Fac. Agron. 2014, 31, 341–361. [Google Scholar]
- Olivares, B.; Rey, J.C.; Lobo, D.; Navas-Cortés, J.A.; Gómez, J.A.; Landa, B.B. Fusarium Wilt of Bananas: A Review of Agro-Environmental Factors in the Venezuelan Production System Affecting Its Development. Agronomy 2021, 11, 986. [Google Scholar] [CrossRef]
- Paredes, F.; Rey, J.; Lobo, D.; Galvis-Causil, S.; Olivares, B. The relationship between the normalized difference vegetation index, rainfall, and potential evapotranspiration in a banana plantation of Venezuela. STJSSA 2021, 18, 58–64. [Google Scholar]
- Rey, J.C.; Martínez-Solórzano, G.; Ramírez, H.; Pargas-Pichardo, R. Cavendish Banana Wilt, and its relationship with agroecological conditions in a lacustrine plain of Venezuela. Agron. Trop. 2020, 70, 1–12. (In Spanish) [Google Scholar] [CrossRef]
- Perry, D.A. Pathogenicity of Monographella nivalis to spring barley. Trans. Br. Mycol. Soc. 1986, 86, 287–293. [Google Scholar] [CrossRef]
- Gawlik, J.; Guz, T. Differentiation of the secondary transformations of peat-muck soils in the Wieprz-Krzna canal. In Soils and Climate of the Lublin Region; Materials of the Scientific Conference of the Lublin Scientific Society: Lublin, Poland, 1995; pp. 72–76. (In Polish) [Google Scholar]
- Okruszko, H. Classification system of hydrogenic soils in Poland. Taxonomy of soils and hydrogenic habitats applied in Poland. Bibl. Wiad. INUZ 1994, 84, 5–27. (In Polish) [Google Scholar]
- Król, M.; Kobus, J. Oxidation of elemental and organic sulfur (from cysteine) by barley rhizosphere microorganisms. Puławski’s Diary Work. By Inst. Soil Sci. Plant Cultiv. State Res. Inst. 1992, 101, 109–122. (In Polish) [Google Scholar]
- Nelson, P.E.; Toussoun, T.A.; Morasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; The Pennsylvania State University Press: University Park, PA, USA, 1983. [Google Scholar]
- O’Donnell, K.; Rooney, A.P.; Proctor, R.H.; Brown, D.W.; McCormick, S.P.; Ward, T.J.; Frandsen, R.J.; Lysøe, E.; Rehner, S.A.; Aoki, T.; et al. Phylogenic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013, 52, 20–31. [Google Scholar] [CrossRef]
- Aoki, T.; O’Donnell, K.; Geiser, D.M. Systematics of key phytopathogenic Fusarium species: Current status and future challenges. J. Gen. Plant Pathol. 2014, 80, 189–201. [Google Scholar] [CrossRef]
- Summerell, B.A. Resolving Fusarium: Current status of the genus. Annu. Rev. Phytopathol. 2019, 57, 323–339. [Google Scholar] [CrossRef]
- Villani, A.; Proctor, R.H.; Kim, H.-S.; Brown, D.W.; Logrieco, A.F.; Amatulli, M.T.; Moretti, A.; Susca, A. Variation in secondary metabolite production potential in the Fusarium incarnatum-equiseti species complex revealed by comparative analysis of 13 genomes. BMC Genom. 2019, 20, 314. [Google Scholar] [CrossRef]
- Xia, J.W.; Sandoval-Denis, M.; Crous, P.W.; Zhang, X.G.; Lombard, L. Numbers to names—Restyling the Fusarium incarnatum-equiseti species complex. Persoonia 2019, 43, 186–221. [Google Scholar] [CrossRef]
- Geiser, D.M.; Al-Hatmi, A.; Aoki, T.; Arie, T.; Balmas, V.; Barnes, I.; Bergstrom, G.C.; Bhattacharyya, M.K.K.; Blomquist, C.L.; Bowden, R.; et al. Phylogenomic analysis of a 55.1 kb 19-gene dataset resolves a monophyletic Fusarium that includes the Fusarium solani species complex. Phytopathology 2021, 111, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Generic concepts in Nectriaceae. Stud. Mycol. 2015, 80, 189–245. [Google Scholar] [CrossRef] [Green Version]
- Crous, P.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.; Schroers, H.-J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef]
- Guan, Y.M.; Ma, Y.Y.; Jin, Q.; Wang, Q.X.; Liu, N.; Fu, Y.P.; Zhang, Y.Y.; Li, Y. Multi-locus phylogeny and taxonomy of the fungal complex associated with rusty root rot of Panax ginseng in China. Front. Microbiol. 2020, 11, 618942. [Google Scholar] [CrossRef]
- Ramon, L.C.; Stroup, W.W.; Freund, R.J. SAS for Linear Models, 4th ed.; SAS Institute Incorporated: Cary, NC, USA, 2009. [Google Scholar]
- Dobosz, M. Computer Aided Statistical Analysis of Research Results; Academic Publishing House EXIT: Warsaw, Poland, 2001. (In Polish) [Google Scholar]
- Frątczak, E.; Pęczkowski, M.; Sienkiewicz, K.; Skaskiewicz, K. Basics in Statistics with the SAS System, version 9.1.; SGH: Warsaw, Poland, 2005. (In Polish) [Google Scholar]
- Stanisz, A. Biostatistics; Jagiellonian University Publishing House: Krakow, Poland, 2005. (In Polish) [Google Scholar]
- Trojan, P. General Ecology; Polish Scientific Publishing House: Warsaw, Poland, 1975. (In Polish) [Google Scholar]
- Szewczyk, W. Soil fungi communities from young Scots pine plantations affected with root rot. Acta Mycol. 2007, 42, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Krebs, C.J. Ecology. The Experimental Analysis of Distribution and Abundance, 4th ed.; Harper-Collins Publish: New York, NY, USA, 1994. [Google Scholar]




| Complexes 1/Genera 2 | Number of Records (Frequency) | % of Total Records |
|---|---|---|
| Fusarium1 | 1447 | 82.9 |
| Cylindrocarpon2 | 6 | 0.3 |
| Ilyonectria1 | 27 | 1.6 |
| Fusicolla2 | 9 | 0.5 |
| Microdochium2 | 256 | 14.7 |
| Total | 1745 | 100.0 |
| Fungal Species 1/Complex 2 | Non-Fertilized Clovers (nfC) | Fertilized Clovers (fC) | Non-Fertilized Grasses (nfC) | Fertilized Grasses (fG) | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Isolates | % | Number of Isolates | % | Number of Isolates | % | Number of Isolates | % | Number of Isolates | % | |
| F. culmorum (W.G. Smitch) Sacc. 1 FSAMSC 2 | 22 | 3.9 | 32 | 6.0 | 63 | 19.3 | 40 | 12.8 | 157 | 9.0 |
| F. graminearum Schwabe FSAMSC | 14 | 2.5 | 0 | 0.0 | 2 | 0.6 | 3 | 1.0 | 19 | 1.1 |
| F. poae (Peck) Wollenw. FSAMSC | 0 | 0.0 | 0 | 0.0 | 2 | 0.6 | 0 | 0.0 | 2 | 0.1 |
| F. sambucinum Fuckel FSAMSC | 33 | 5.8 | 22 | 4.1 | 8 | 2.4 | 6 | 1.9 | 69 | 3.9 |
| F. sporotrichioides Sherb. FSAMSC | 33 | 5.8 | 28 | 5.2 | 18 | 5.5 | 53 | 16.9 | 132 | 7.6 |
| F. avenaceum (Fr.) Sacc. FTSC | 98 | 17.2 | 39 | 7.3 | 23 | 7.0 | 40 | 12.8 | 200 | 11.4 |
| F. tricinctum (Corda) Sacc. FTSC | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 |
| F. equiseti (Corda) Sacc. FIESC | 3 | 0.5 | 6 | 1.1 | 3 | 0.9 | 0 | 0.0 | 12 | 0.7 |
| F. incarnatum (Desm.) Sacc. FIESC | 0 | 0.0 | 0 | 0.0 | 1 | 0.3 | 4 | 1.3 | 5 | 0.3 |
| F. oxysporum Schlecht. emand. Sny. and Hans. FOSC | 241 | 42.3 | 302 | 56.2 | 128 | 39.1 | 100 | 31.9 | 771 | 44.1 |
| F. sacchari (Butler) W. Gams FFSC | 15 | 2.6 | 4 | 0.7 | 0 | 0.0 | 2 | 0.6 | 21 | 1.2 |
| F. solani (Mart.) Sacc. FSSC | 2 | 0.4 | 6 | 1.1 | 1 | 0.3 | 0 | 0.0 | 9 | 0.5 |
| F. lateritium Nees ex Link FLSC | 21 | 3.7 | 8 | 1.5 | 4 | 1.2 | 8 | 2.6 | 41 | 2.3 |
| F. camptoceras Wollenw. & Reinking FCAMSC | 1 | 0.2 | 4 | 0.7 | 2 | 0.6 | 1 | 0.3 | 8 | 0.5 |
| C. didymum (Hartig) Wollenw. | 0 | 0.0 | 0 | 0.0 | 1 | 0.3 | 3 | 1.0 | 4 | 0.2 |
| C. magnusianum (Sacc.) Wollenw. | 0 | 0.0 | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 | 2 | 0.1 |
| Ilyonectria/Cylindrocarpon destructans (Zinssm.) Rossman, L. Lombard and Crous IRSC | 6 | 1.1 | 1 | 0.2 | 13 | 4.0 | 7 | 2.2 | 27 | 1.5 |
| Fusicolla aquaeductuum (Radlk. and Rabenh.) Gräfenhan, Seifert and Schroers | 0 | 0.0 | 2 | 0.4 | 1 | 0.3 | 5 | 1.6 | 8 | 0.5 |
| Fusicolla merismoides (Corda) Gräfenhan, Seifert and Schroers | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 |
| Microdochium nivale (Fr.) Samuels and I.C. Hallett | 78 | 13.7 | 80 | 14.9 | 57 | 17.4 | 41 | 13.1 | 256 | 14.7 |
| Total | 570 a * | 100 | 537 a | 100 | 327 a | 100 | 313 a | 100 | 1747 | 100 |
| C | Species 1/Complex 2 | Ectorhizosphere (Ec) | Rhizoplane (Rp) | Endorhizosphere (Ed) | Total of Analyses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AI | AII | AIII | AI | AII | AIII | AI | AII | AIII | Ec | Rp | Ed | ||
| nfC | F. sambucinum1 FSAMSC 2 | 9.2 | − | − | 6.1 | − | − | 16.3 | − | − | 8.6 | 4.1 | 7.9 |
| F. sporotrichioides FSAMSC | 9.2 | − | − | 13.6 | − | − | 11.6 | 2.9 | − | 8.6 | 9.2 | 6.7 | |
| Fusarium avenaceum FTSC | 29.2 | − | − | 12.1 | 3.8 | − | 13.9 | 2.9 | − | 27.1 | 9.2 | 7.9 | |
| F. oxysporum FOSC | 36.9 | 100.0 | − | 48.5 | 46.2 | − | 41.9 | 94.1 | − | 41.4 | 44.9 | 56.2 | |
| Microdochium nivale | − | − | − | 6.1 | 38.5 | 100.0 | − | − | 100.0 | − | 20.4 | 13.5 | |
| nfG | F. culmorum FSAMSC | 33.3 | 33.3 | 50.0 | 11.1 | 16.7 | − | − | − | 20.0 | 38.5 | 11.6 | 4.3 |
| F. poae FSAMSC | 6.7 | − | 12.5 | − | − | − | − | − | − | 7.7 | − | − | |
| F. sambucinum FSAMSC | 20.0 | − | − | − | − | − | − | − | − | 11.5 | − | − | |
| F. sporotrichioides FSAMSC | − | − | − | 11.1 | − | − | 25.0 | − | − | − | 7.0 | 10.0 | |
| F. avenaceum FTSC | 13.3 | 33.3 | − | 7.4 | 33.3 | − | − | − | − | 11.5 | 14.0 | − | |
| F. oxysporum FOSC | 20.0 | − | − | 44.4 | 16.7 | − | 53.6 | 100.0 | 6.7 | 11.5 | 32.6 | 61.4 | |
| F. camptoceras FCAMSC | − | 33.3 | − | − | − | − | − | − | − | 3.8 | − | − | |
| Ilyonectria/Cylindrocarpon destructans IRSC | 6.7 | − | − | − | − | − | 17.9 | − | 46.7 | 3.8 | − | 17.1 | |
| F. aquaeductuum | − | − | 12.5 | − | − | − | − | − | − | 3.8 | − | − | |
| Microdochium nivale | − | − | 25.0 | 14.8 | 33.3 | 100.0 | − | − | 26.7 | 7.7 | 27.9 | 5.7 | |
| fC | F. sambucinum FSAMSC | − | − | − | 0.9 | 5.9 | − | 18.0 | − | − | − | 1.4 | 11.7 |
| F. sporotrichioides FSAMSC | 23.3 | 2.9 | 18.2 | − | 11.8 | − | − | − | − | 13.3 | 1.4 | − | |
| F. avenaceum FTSC | 10.0 | 2.9 | 9.1 | − | − | − | 26.0 | − | − | 6.7 | − | 16.9 | |
| F. oxysporum FOSC | 53.3 | 82.4 | 63.6 | 85.2 | 58.8 | 42.9 | 46.0 | 96.3 | − | 68.0 | 79.9 | 63.6 | |
| F. aquaeductuum | − | − | − | − | 11.8 | − | − | − | − | − | 1.4 | − | |
| Microdochium nivale | − | − | − | 7.8 | 11.8 | 57.1 | − | − | − | − | 10.8 | − | |
| fG | F. culmorum FSAMSC | − | − | − | 22.9 | − | − | 35.7 | 8.6 | − | − | 11.3 | 20.6 |
| F. sambucinum FSAMSC | − | − | − | 5.7 | − | − | 3.6 | − | − | − | 2.8 | 1.6 | |
| F. sporotrichioides FSAMSC | 3.0 | − | − | 14.3 | − | 9.1 | 7.1 | − | − | 25.0 | 9.9 | 3.2 | |
| F. avenaceum FTSC | 24.0 | 36.4 | − | 2.9 | 14.3 | 13.6 | 32.1 | − | − | 27.8 | 8.5 | 14.3 | |
| F. oxysporum FOSC | 16.0 | 36.4 | − | 17.1 | 71.4 | 36.4 | 7.1 | 91.4 | − | 22.2 | 33.8 | 54.0 | |
| F. lateritium FLSC | 12.0 | 9.1 | − | 2.9 | − | 9.1 | − | − | − | 11.1 | 4.2 | − | |
| F. camptoceras FCAMSC | 4.0 | − | − | − | − | − | − | − | − | 2.8 | − | − | |
| Ilyonectria/Cylindrocarpon destructans IRSC | − | − | − | − | − | − | 14.3 | − | − | − | − | 6.3 | |
| Fusicolla aquaeductuum | − | 18.2 | − | − | − | − | − | − | − | 5.6 | − | − | |
| Microdochium nivale | − | − | − | 34.3 | 14.3 | 27.3 | − | − | − | − | 28.2 | − | |
| C | Species 1/Complex 2 | Ectorhizosphere (Ec) | Rhizoplane (Rp) | Endorhizosphere (Ed) | Total of Analyses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AI | AII | AIII | AI | AII | AIII | AI | AII | AIII | Ec | Rp | Ed | ||
| nfC | F. culmorum1 FSAMSC 2 | 4.6 | − | − | 6.1 | − | − | 4.7 | − | − | 4.3 | 4.1 | 2.2 |
| F. graminearum FSAMSC | 1.5 | − | − | 1.5 | 7.7 | − | 2.3 | − | − | 1.4 | 3.1 | 1.1 | |
| F. equiseti FIESC | − | − | − | 3.0 | − | − | − | − | − | − | 2.0 | − | |
| F. lateritium FLSC | − | − | − | 3.0 | 3.8 | − | 7.0 | − | − | − | 3.1 | 3.4 | |
| F. camptoceras FCAMSC | − | − | − | − | − | − | 2.3 | − | − | − | − | 1.1 | |
| Ilyonectria/Cylindrocarpon destructans IRSC | 9.2 | − | − | − | − | − | − | − | − | 8.6 | − | − | |
| nfG | F. graminearum FSAMSC | − | − | − | − | − | − | 3.6 | − | − | − | − | 1.4 |
| F. equiseti FIESC | − | − | − | 7.4 | − | − | − | − | − | − | 4.7 | − | |
| F. incarnatum FIESC | − | − | − | 3.7 | − | − | − | − | − | − | 2.3 | − | |
| fC | F. culmorum FSAMSC | 6.7 | 8.8 | − | 2.6 | − | − | 10.0 | − | − | 6.7 | 2.2 | 6.5 |
| F. sacchari FFSC | − | 2.9 | 9.1 | − | − | − | − | − | − | 2.7 | − | − | |
| F. lateritium FLSC | − | − | − | 3.5 | − | − | − | − | − | − | 2.9 | − | |
| F. camptoceras FCAMSC | 6.7 | − | − | − | − | − | − | − | − | 2.7 | − | − | |
| Ilyonectria/Cylindrocarpon destructans IRSC | − | − | − | − | − | − | − | 3,7 | − | − | − | 1.3 | |
| fG | F. incarnatum FIESC | 8.0 | − | − | − | − | − | − | − | − | 5.6 | − | − |
| F. sacchari FFSC | − | − | − | − | − | 4.5 | − | − | − | − | 1.4 | − | |
| Combination | Compared Communities | Analyses (A) | Total of Analyses | Mean of Analyses | ||
|---|---|---|---|---|---|---|
| I | II | III | ||||
| nfC | Ec—Rp | 60.0 | 20.0 | 0.0 | 60.0 | 26.7 |
| Rp—Ed | 63.6 | 33.3 | 100.0 | 72.7 | 65.7 | |
| Ec—Ed | 60.0 | 33.3 | 0.0 | 54.5 | 31.1 | |
| nfG | Ec—Rp | 30.0 | 40.0 | 25.0 | 33.3 | 31.7 |
| Rp—Ed | 22.2 | 25.0 | 25.0 | 44.4 | 24.1 | |
| Ec—Ed | 25.0 | 0.0 | 33.3 | 36.4 | 19.4 | |
| fC | Ec—Rp | 25.0 | 25.0 | 20.0 | 30.0 | 23.3 |
| Rp—Ed | 50.0 | 16.7 | 0.0 | 33.3 | 22.2 | |
| Ec—Ed | 50.0 | 16.7 | 0.0 | 37.5 | 22.2 | |
| fG | Ec—Rp | 44.4 | 40.0 | 0.0 | 36.4 | 28.1 |
| Rp—Ed | 62.5 | 25.0 | 0.0 | 55.6 | 29.2 | |
| Ec—Ed | 33.3 | 20.0 | 0.0 | 30.0 | 17.8 | |
| Plant Combination | Analysis (A) | Ectorhizosphere (Ec) | Rhizoplane (Rp) | Endorhizosphere (Ed) | Total of Analyses | ||
|---|---|---|---|---|---|---|---|
| Ec | Rp | Ed | |||||
| nfC | I | 0.750 | 0.719 | 0.766 | 0.731 | 0.734 | 0.650 |
| II | 0.000 | 0.630 | 0.112 | ||||
| III | − | 0.000 | 0.000 | ||||
| nfG | I | 0.782 | 0.743 | 0.617 | 0.796 | 0.776 | 0.578 |
| II | 0.667 | 0.722 | 0.000 | ||||
| III | 0.656 | 0.684 | 0.667 | ||||
| fC | I | 0.642 | 0.266 | 0.678 | 0.510 | 0.349 | 0.548 |
| II | 0.311 | 0.609 | 0.071 | ||||
| III | 0.545 | 0.490 | − | ||||
| fG | I | 0.765 | 0.776 | 0.737 | 0.792 | 0.774 | 0.640 |
| II | 0.694 | 0.449 | 0.157 | ||||
| III | − | 0.756 | − | ||||
| Fungal Species 1/Complex 2 | Microenvironment | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Ectorhizosphere | Rhizoplane | Endorhizosphere | ||||||
| Number | % | Number | % | Number | % | Number | % | |
| F. culmorum1 FSAMSC 2 | 4 | 0.5 | 0 | 0.0 | 1 | 0.1 | 5 | 0.6 |
| F. graminearum FSAMSC | 0 | 0.0 | 4 | 0.5 | 0 | 0.0 | 4 | 0.5 |
| F. poae FSAMSC | 2 | 0.2 | 0 | 0.0 | 0 | 0.0 | 2 | 0.2 |
| F. sambucinum FSAMSC | 9 | 1.1 | 8 | 0.9 | 17 | 2.0 | 34 | 4.0 |
| F. sporotrichioides FSAMSC | 25 | 2.9 | 21 | 2.5 | 15 | 1.8 | 61 | 7.1 |
| F. avenaceum FTSC | 3 | 0.4 | 2 | 0.2 | 2 | 0.2 | 7 | 0.7 |
| F. equiseti FIESC | 18 | 2.1 | 20 | 2.3 | 23 | 2.7 | 61 | 7.1 |
| F. incarnatum FIESC | 2 | 0.2 | 1 | 0.1 | 0 | 0.0 | 3 | 0.4 |
| F. oxysporum FOSC | 91 | 10.6 | 193 | 22.5 | 176 | 20.5 | 460 | 53.7 |
| F. sacchari FFSC | 2 | 0.2 | 1 | 0.1 | 0 | 0.0 | 3 | 0.4 |
| F. lateritium FLSC | 1 | 0.1 | 3 | 0.4 | 2 | 0.2 | 6 | 0.7 |
| F. camptoceras FCAMSC | 37 | 4.3 | 21 | 2.5 | 27 | 3.2 | 85 | 9.9 |
| Ilyonectria/Cylindrocarpon destructans IRSC | 2 * | 0.2 | 67 | 7.8 | 16 | 1.9 | 85 | 9.9 |
| Fusicolla aquaeductuum | 7 | 0.8 | 0 | 0.0 | 17 | 2.0 | 24 | 2.8 |
| Microdochium nivale | 4 | 0.5 | 10 | 1.2 | 3 | 0.4 | 17 | 2.0 |
| Total | 207.0 | 24.2 | 351.0 | 41.0 | 299.0 | 34.9 | 857.0 | 100.0 |
| Statistics | Value | Probability | ||||||
| Pearson’s chi-square | 329.55 | <0.0001 | ||||||
| Likelihood-ratio chi-square | 345.65 | <0.0001 | ||||||
| Mantel–Haenszel chi-square | 9.59 | 0.002 | ||||||
| Φ-Yule’s coefficient | 0.43 | |||||||
| C-Pearson contingency coefficient | 0.40 | |||||||
| Cramer’s V coefficient | 0.22 | |||||||
| Combination | % Organic Matter | % N Total | mg CaO in 100 g of Soil | mg w 100 g of Soil Acc. to Egner | Milligram Equivalents Ca in 100 g of Soil | pH 9 (KCl) | |
|---|---|---|---|---|---|---|---|
| P2O5 | K2O | ||||||
| Non-fertilized soil | 51.59 | 1.78 | 95.03 | 13.70 | 7.19 | 33.87 | 4.40 |
| Fertilized soil | 72.78 | 2.25 | 147.78 | 16.00 | 9.90 | 55.68 | 5.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korniłłowicz-Kowalska, T.; Wojdyło-Kotwica, B.; Bohacz, J.; Możejko, M. Occurrence and Distribution of Fusarium Communities in the Root Zone in a Post-Bog Permanent Meadow in Relation to Mineral Fertilization and Growing Seasons. Pathogens 2022, 11, 341. https://doi.org/10.3390/pathogens11030341
Korniłłowicz-Kowalska T, Wojdyło-Kotwica B, Bohacz J, Możejko M. Occurrence and Distribution of Fusarium Communities in the Root Zone in a Post-Bog Permanent Meadow in Relation to Mineral Fertilization and Growing Seasons. Pathogens. 2022; 11(3):341. https://doi.org/10.3390/pathogens11030341
Chicago/Turabian StyleKorniłłowicz-Kowalska, Teresa, Bernadeta Wojdyło-Kotwica, Justyna Bohacz, and Michał Możejko. 2022. "Occurrence and Distribution of Fusarium Communities in the Root Zone in a Post-Bog Permanent Meadow in Relation to Mineral Fertilization and Growing Seasons" Pathogens 11, no. 3: 341. https://doi.org/10.3390/pathogens11030341