Illuminating Human Norovirus: A Perspective on Disinfection of Water and Surfaces Using UVC, Norovirus Model Organisms, and Radiation Safety Considerations
Abstract
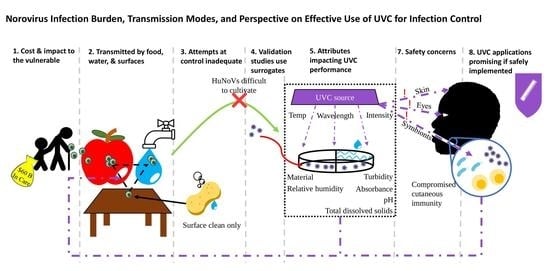
:1. Background
2. Transmission of HuNoVs
3. HuNoV Control Programs
4. Surrogates: Considerations for Picking Norovirus Model Organisms for UVC Disinfection Studies
5. UVC Disinfection Studies That Utilized Norovirus Surrogates
6. Considerations for Effective Use of UVC in Surface Disinfection
7. Considerations for Conducting Water Disinfection Using UVC
8. Radiation Safety Considerations
9. The Promise of UVC for Norovirus
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, K.Y.; Kaufman, S.S.; Nagata, B.M.; Chaimongkol, N.; Kim, D.Y.; Levenson, E.A.; Tin, C.M.; Yardley, A.B.; Johnson, J.A.; Barletta, A.B.F.; et al. Human norovirus targets enteroendocrine epithelial cells in the small intestine. Nat. Commun. 2020, 11, 2759. [Google Scholar] [CrossRef] [PubMed]
- Karst, S.M. Pathogenesis of Noroviruses, Emerging RNA Viruses. Viruses 2010, 2, 748–781. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; De Graaf, M.; Parra, G.I.; Chan, M.C.-W.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Bidalot, M.; Théry, L.; Kaplon, J.; De Rougemont, A.; Ambert-Balay, K. Emergence of new recombinant noroviruses GII.p16-GII.4 and GII.p16-GII.2, France, winter 2016 to 2017. Eurosurveillance 2017, 22, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, H.; Magalis, B.R.; Pond, S.L.K.; Volz, E.M. Molecular Evolution of Human Norovirus GII.2 Clusters. Front. Microbiol. 2021, 12, 655567. [Google Scholar] [CrossRef]
- Medici, M.C.; Tummolo, F.; Martella, V.; De Conto, F.; Arcangeletti, M.C.; Pinardi, F.; Ferraglia, F.; Chezzi, C.; Calderaro, A. Emergence of novel recombinant GII.P16_GII.2 and GII. P16_GII.4 Sydney 2012 norovirus strains in Italy, winter 2016/2017. New Microbiol. 2018, 41, 71–72. [Google Scholar]
- Tohma, K.; Lepore, C.J.; Siltz, L.; Parra, G.I. Phylogenetic Analyses Suggest that Factors Other Than the Capsid Protein Play a Role in the Epidemic Potential of GII.2 Norovirus. mSphere 2017, 2, e00187-17. [Google Scholar] [CrossRef] [Green Version]
- Niendorf, S.; Jacobsen, S.; Faber, M.; Eis-Hübinger, A.M.; Hofmann, J.; Zimmermann, O.; Höhne, M.; Bock, C.-T. Steep rise in norovirus cases and emergence of a new recombinant strain GII.P16-GII.2, Germany, winter 2016. Eurosurveillance 2017, 22, 30447. [Google Scholar] [CrossRef]
- Cheung, S.K.C.; Kwok, K.; Zhang, L.Y.; Mohammad, K.N.; Lui, G.C.Y.; Lee, N.; Nelson, E.A.S.; Lai, R.W.M.; Leung, T.K.; Chan, P.K.S.; et al. Higher Viral Load of Emerging Norovirus GII.P16-GII.2 than Pandemic GII.4 and Epidemic GII.17, Hong Kong, China. Emerg. Infect. Dis. 2019, 25, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, S.M.; Lopman, B.A.; Ozawa, S.; Hall, A.J.; Lee, B.Y. Global Economic Burden of Norovirus Gastroenteritis. PLoS ONE 2016, 11, e0151219. [Google Scholar] [CrossRef] [Green Version]
- Vinjé, J. Advances in Laboratory Methods for Detection and Typing of Norovirus. J. Clin. Microbiol. 2015, 53, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockey, N.; Young, S.; Kohn, T.; Pecson, B.M.; Wobus, C.E.; Raskin, L.; Wigginton, K.R. UV Disinfection of Human Norovirus: Evaluating Infectivity Using a Genome-Wide PCR-Based Approach. Environ. Sci. Technol. 2020, 54, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Green, K.Y. Norovirus infection in immunocompromised hosts. Clin. Microbiol. Infect. 2014, 20, 717–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, D.C.; Vinje, J.; Szilagyi, P.G.; Edwards, K.M.; Staat, M.A.; Weinberg, G.; Hall, C.B.; Chappell, J.; Bernstein, D.I.; Curns, A.T.; et al. Norovirus and Medically Attended Gastroenteritis in U.S. Children. N. Engl. J. Med. 2013, 368, 1121–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.P.; Edmunds, W.J.; Pebody, R.; Brown, D.W.; Lopman, B.A. Deaths from Norovirus among the Elderly, England and Wales. Emerg. Infect. Dis. 2008, 14, 1546–1552. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yoshizumi, S.; Kogawa, S.; Takahashi, T.; Ueki, Y.; Shinohara, M.; Mizukoshi, F.; Tsukagoshi, H.; Sasaki, Y.; Suzuki, R.; et al. Molecular Evolution of the Capsid Gene in Norovirus Genogroup I. Sci. Rep. 2015, 5, 13806. [Google Scholar] [CrossRef] [Green Version]
- Esposito, S.; Principi, N. Norovirus Vaccine: Priorities for Future Research and Development. Front. Immunol. 2020, 11, 1383. [Google Scholar] [CrossRef]
- Zhou, H.-L.; Chen, L.-N.; Wang, S.-M.; Tan, M.; Qiu, C.; Qiu, T.-Y.; Wang, X.-Y. Prevalence and Evolution of Noroviruses between 1966 and 2019, Implications for Vaccine Design. Pathogens 2021, 10, 1012. [Google Scholar] [CrossRef]
- Schneider, K.R.; Goodrich, R.M.; Mahovic, M.J.; Shukla, R. Preventing Foodborne Illness: Norovirus. EDIS 2019, 2005, 1–4. [Google Scholar] [CrossRef]
- Cannon, J.L.; Barclay, L.; Collins, N.R.; Wikswo, M.E.; Castro, C.J.; Magaña, L.C.; Gregoricus, N.; Marine, R.; Chhabra, P.; Vinjé, J. Genetic and Epidemiologic Trends of Norovirus Outbreaks in the United States from 2013 to 2016 Demonstrated Emergence of Novel GII.4 Recombinant Viruses. J. Clin. Microbiol. 2017, 55, 2208–2221. [Google Scholar] [CrossRef] [Green Version]
- Farkas, T. Rhesus enteric calicivirus surrogate model for human norovirus gastroenteritis. J. Gen. Virol. 2015, 96, 1504–1514. [Google Scholar] [CrossRef]
- Barker, J.; Vipond, I.B.; Bloomfield, S.F. Effects of cleaning and disinfection in reducing the spread of Norovirus contamination via environmental surfaces. J. Hosp. Infect. 2004, 58, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Canales, R.A.; Reynolds, K.A.; Wilson, A.M.; Fankem, S.L.; Weir, M.H.; Rose, J.B.; Abd-Elmaksoud, S.; Gerba, C.P. Modeling the role of fomites in a norovirus outbreak. J. Occup. Environ. Hyg. 2019, 16, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Boxman, I.; Dijkman, R.; Verhoef, L.; Maat, A.; Van Dijk, G.; Vennema, H.; Koopmans, M. Norovirus on Swabs Taken from Hands Illustrate Route of Transmission: A Case Study. J. Food Prot. 2009, 72, 1753–1755. [Google Scholar] [CrossRef] [PubMed]
- Kwan, H.S.; Chan, P.K.S.; Chan, M.C.W. Chapter 2—Overview of Norovirus as a Foodborne Pathogen. In The Norovirus; Chan, P.K.S., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 21–30. ISBN 978-0-12-804177-2. [Google Scholar]
- Maunula, L.; Miettinen, I.T.; Von Bonsdorff, C.-H. Norovirus Outbreaks from Drinking Water. Emerg. Infect. Dis. 2005, 11, 1716–1721. [Google Scholar] [CrossRef]
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef] [Green Version]
- ECDC Facts about Norovirus. Available online: https://www.ecdc.europa.eu/en/norovirus-infection/facts (accessed on 1 December 2021).
- Cromeans, T.L.; Kahler, A.M.; Hill, V.R. Inactivation of Adenoviruses, Enteroviruses, and Murine Norovirus in Water by Free Chlorine and Monochloramine. Appl. Environ. Microbiol. 2010, 76, 1028–1033. [Google Scholar] [CrossRef] [Green Version]
- Cleveland Clinic Norovirus: Symptoms, Causes, Treatments. Available online: https://my.clevelandclinic.org/health/diseases/17703-norovirus (accessed on 1 December 2021).
- Brunette, G.W. CDC Yellow Book 2018: Health Information for International Travel; Oxford University Press: Oxford, UK, 2017; Volume 66, pp. 1157–1158. ISBN 9780190628611. [Google Scholar]
- Koutchma, T.; Popović, V. Chapter 5—UV Light-Emitting Diodes (LEDs) and Food Safety. In Ultraviolet LED Technology for Food Applications; Koutchma, T., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 91–117. ISBN 978-0-12-817794-5. [Google Scholar]
- Koutchma, T. UV Light for Processing Foods. Ozone Sci. Eng. 2008, 30, 93–98. [Google Scholar] [CrossRef]
- MacCannell, T.; Umscheid, C.A.; Agarwal, R.K.; Lee, I.; Kuntz, G.; Stevenson, K.B. Guideline for the Prevention and Control of Norovirus Gastroenteritis Outbreaks in Healthcare Settings. Infect. Control Hosp. Epidemiol. 2011, 32, 939–969. [Google Scholar] [CrossRef] [Green Version]
- Barclay, L.; Park, G.; Vega, E.; Hall, A.; Parashar, U.; Vinje, J.; Lopman, B. Infection control for norovirus. Clin. Microbiol. Infect. 2014, 20, 731–740. [Google Scholar] [CrossRef] [Green Version]
- Vinnard, C.; Lee, I.; Linkin, D.R. Successful control of a norovirus outbreak among attendees of a hospital teaching conference. Am. J. Infect. Control 2012, 40, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q. Epidemic and control strategy on nosocomial outbreak of norovirus gastroenteritis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2008, 30, 614–617. [Google Scholar] [PubMed]
- Georgiadou, S.P.; Loukeris, D.; Smilakou, S.; Daikos, G.L.; Sipsas, N.V. Effective control of an acute gastroenteritis outbreak due to norovirus infection in a hospital ward in Athens, Greece, April 2011. Eurosurveillance 2011, 16, 19915. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Abarca, B.I.; Goulter, R.; Arbogast, J.W.; Leslie, R.A.; Green, K.; Jaykus, L. Efficacy of alcohol-based hand sanitizers against human norovirus using RNase-RT-qPCR with validation by human intestinal enteroid replication. Lett. Appl. Microbiol. 2020, 71, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Miki, M.; Hitomi, J.; Tokuda, H.; Katayama, K.; Kubota, H.; Todaka-Takai, R. Effects of disinfectants against norovirus virus-like particles predict norovirus inactivation. Microbiol. Immunol. 2016, 60, 609–616. [Google Scholar] [CrossRef]
- Crawford, M. How Clean Is Clean? Chemistry Can Damage Medical Equipment In the Quest to Meet Stringent Guidelines. Biomed. Instrum. Technol. 2014, 48, 260–263. [Google Scholar] [CrossRef]
- Tyan, K.; Jin, K.; Kang, J. Novel colour additive for bleach disinfectant wipes reduces corrosive damage on stainless steel. J. Hosp. Infect. 2019, 103, 227–230. [Google Scholar] [CrossRef]
- Buonanno, M.; Welch, D.; Shuryak, I.; Brenner, D.J. Far-UVC light (222 nm) efficiently and safely inactivates airborne human coronaviruses. Sci. Rep. 2020, 10, 10285. [Google Scholar] [CrossRef]
- Hessling, M.; Haag, R.; Sieber, N.; Vatter, P. The Impact of Far-UVC Radiation (200-230 Nm) on Pathogens, Cells, Skin, and Eyes—A Collection and Analysis of a Hundred Years of Data. GMS Hyg. Infect. Control 2021, 16, 1–17. [Google Scholar] [CrossRef]
- Minegishi, K.; Aikawa, C.; Furukawa, A.; Watanabe, T.; Nakano, T.; Ogura, Y.; Ohtsubo, Y.; Kurokawa, K.; Hayashi, T.; Maruyama, F.; et al. Complete Genome Sequence of a Propionibacterium acnes Isolate from a Sarcoidosis Patient. Genome Announc. 2013, 1, e00016-12. [Google Scholar] [CrossRef] [Green Version]
- Dumas, O.; Varraso, R.; Boggs, K.M.; Quinot, C.; Zock, J.-P.; Henneberger, P.K.; Speizer, F.E.; Le Moual, N.; Camargo, C.A., Jr. Association of Occupational Exposure to Disinfectants with Incidence of Chronic Obstructive Pulmonary Disease Among US Female Nurses. JAMA Netw. Open 2019, 2, e1913563. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, W.; Böhm, K.H.; Richter, B. [Studies on the corrosive action of some disinfectants suitable for aerosol-disinfection (author’s transl)]. Zentralbl. Bakteriol. Mikrobiol. Hyg. B 1981, 173, 374–381. [Google Scholar] [PubMed]
- Cao, C. Chemical Disinfectants for Inactivation of Human Norovirus Surrogates. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2013. [Google Scholar]
- Poschetto, L.F.; Ike, A.; Papp, T.; Mohn, U.; Böhm, R.; Marschang, R.E. Comparison of the Sensitivities of Noroviruses and Feline Calicivirus to Chemical Disinfection under Field-Like Conditions. Appl. Environ. Microbiol. 2007, 73, 5494–5500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baert, L.; Vandekinderen, I.; Devlieghere, F.; Van Coillie, E.; Debevere, J.; Uyttendaele, M. Efficacy of Sodium Hypochlorite and Peroxyacetic Acid to Reduce Murine Norovirus 1, B40-8, Listeria monocytogenes, and Escherichia coli O157:H7 on Shredded Iceberg Lettuce and in Residual Wash Water. J. Food Prot. 2009, 72, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Stoltzfus, G.T.; Zhu, C.; Jung, K.; Wang, Q.; Saif, L.J. Attempts to grow human noroviruses, a sapovirus, and a bovine norovirus in vitro. PLoS ONE 2018, 13, e0178157. [Google Scholar] [CrossRef]
- Keswick, B.H.; Satterwhite, T.K.; Johnson, P.C.; DuPont, H.L.; Secor, S.L.; Bitsura, J.A.; Gary, G.W.; Hoff, J.C. Inactivation of Norwalk virus in drinking water by chlorine. Appl. Environ. Microbiol. 1985, 50, 261–264. [Google Scholar] [CrossRef] [Green Version]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.; Neill, F.H.; Blutt, S.E.; Zeng, X.-L.; Qu, L.; et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef]
- Rönnqvist, M.; Mikkelä, A.; Tuominen, P.; Salo, S.; Maunula, L. Ultraviolet Light Inactivation of Murine Norovirus and Human Norovirus GII: PCR May Overestimate the Persistence of Noroviruses Even When Combined with Pre-PCR Treatments. Food Environ. Virol. 2013, 6, 48–57. [Google Scholar] [CrossRef]
- Manuel, C.S.; Moore, M.D.; Jaykus, L.-A. Predicting human norovirus infectivity—Recent advances and continued challenges. Food Microbiol. 2018, 76, 337–345. [Google Scholar] [CrossRef]
- Sinclair, R.G.; Rose, J.B.; Hashsham, S.A.; Gerba, C.P.; Haas, C. Criteria for Selection of Surrogates Used to Study the Fate and Control of Pathogens in the Environment. Appl. Environ. Microbiol. 2012, 78, 1969–1977. [Google Scholar] [CrossRef] [Green Version]
- Hirneisen, K.A.; Kniel, K.E. Comparing Human Norovirus Surrogates: Murine Norovirus and Tulane Virus. J. Food Prot. 2013, 76, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Hutson, A.M.; Atmar, R.L.; Graham, D.Y.; Estes, M.K. Norwalk Virus Infection and Disease Is Associated with ABO Histo–Blood Group Type. J. Infect. Dis. 2002, 185, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Jiang, X. Norovirus Gastroenteritis, Carbohydrate Receptors, and Animal Models. PLoS Pathog. 2010, 6, e1000983. [Google Scholar] [CrossRef] [PubMed]
- Farkas, T.; Cross, R.W.; Hargitt, E., 3rd; Lerche, N.W.; Morrow, A.L.; Sestak, K. Genetic Diversity and Histo-Blood Group Antigen Interactions of Rhesus Enteric Caliciviruses. J. Virol. 2010, 84, 8617–8625. [Google Scholar] [CrossRef] [Green Version]
- Almand, E.A.; Moore, M.D.; Jaykus, L.-A. Norovirus Binding to Ligands Beyond Histo-Blood Group Antigens. Front. Microbiol. 2017, 8, 2549. [Google Scholar] [CrossRef] [Green Version]
- Varki, A. Multiple Changes in Sialic Acid Biology During Human Evolution. Glycoconj J. 2009, 26, 231–245. [Google Scholar] [CrossRef]
- Stuart, A.D.; Brown, T.D.K. α2,6-Linked sialic acid acts as a receptor for Feline calicivirus. J. Gen. Virol. 2007, 88, 177–186. [Google Scholar] [CrossRef]
- Taube, S.; Perry, J.W.; Yetming, K.; Patel, S.P.; Auble, H.; Shu, L.; Nawar, H.F.; Lee, C.H.; Connell, T.D.; Shayman, J.A.; et al. Ganglioside-Linked Terminal Sialic Acid Moieties on Murine Macrophages Function as Attachment Receptors for Murine Noroviruses. J. Virol. 2009, 83, 4092–4101. [Google Scholar] [CrossRef] [Green Version]
- Richards, G.P. Critical Review of Norovirus Surrogates in Food Safety Research: Rationale for Considering Volunteer Studies. Food Environ. Virol. 2012, 4, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Park, G.W.; Sobsey, M.D. Simultaneous Comparison of Murine Norovirus, Feline Calicivirus, Coliphage MS2, and GII.4 Norovirus to Evaluate the Efficacy of Sodium Hypochlorite Against Human Norovirus on a Fecally Soiled Stainless Steel Surface. Foodborne Pathog. Dis. 2011, 8, 1005–1010. [Google Scholar] [CrossRef]
- Nims, R.; Plavsic, M. Inactivation of Caliciviruses. Pharmaceuticals 2013, 6, 358–392. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Schwab, K.J. Evaluation of Murine Norovirus, Feline Calicivirus, Poliovirus, and MS2 as Surrogates for Human Norovirus in a Model of Viral Persistence in Surface Water and Groundwater. Appl. Environ. Microbiol. 2008, 74, 477–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, P.; Yang, D.; Quigley, C.; Chou, M.; Jiang, X. Inactivation of the Tulane Virus, a Novel Surrogate for the Human Norovirus. J. Food Prot. 2013, 76, 712–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mormann, S.; Heißenberg, C.; Pfannebecker, J.; Becker, B. Tenacity of Human Norovirus and the Surrogates Feline Calicivirus and Murine Norovirus during Long-Term Storage on Common Nonporous Food Contact Surfaces. J. Food Prot. 2015, 78, 224–229. [Google Scholar] [CrossRef]
- Oguma, K.; Rattanakul, S. UV Inactivation of Viruses in Water: Its Potential to Mitigate Current and Future Threats of Viral Infectious Diseases. Jpn. J. Appl. Phys. 2021, 60, 110502. [Google Scholar] [CrossRef]
- Ohmine, T.; Narai, S.; Matsubara, T.; Nomura, T.; Oda, K.; Fukushi, M.; Irie, T.; Komatsu, T.; Tohya, Y.; Sakaguchi, T. Eligibility of Feline Calicivirus for a Surrogate of Human Norovirus in Comparison with Murine Norovirus, Poliovirus and Coxsackievirus. Biocontrol Sci. 2018, 23, 145–149. [Google Scholar] [CrossRef] [Green Version]
- Park, G.W.; Barclay, L.; Macinga, D.; Charbonneau, D.; Pettigrew, C.A.; Vinje, J. Comparative Efficacy of Seven Hand Sanitizers against Murine Norovirus, Feline Calicivirus, and GII.4 Norovirus. J. Food Prot. 2010, 73, 2232–2238. [Google Scholar] [CrossRef]
- Radford, A.D.; Coyne, K.P.; Dawson, S.; Porter, C.J.; Gaskell, R.M. Feline calicivirus. Vet. Res. 2007, 38, 319–335. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Shahbaz, H.; Kim, S.-H.; Lee, M.; Lee, W.; Oh, J.-W.; Lee, D.-U.; Park, J. Inactivation efficiency and mechanism of UV-TiO2 photocatalysis against murine norovirus using a solidified agar matrix. Int. J. Food Microbiol. 2016, 238, 256–264. [Google Scholar] [CrossRef]
- Hewitt, J.; Rivera-Aban, M.; Greening, G. Evaluation of murine norovirus as a surrogate for human norovirus and hepatitis A virus in heat inactivation studies. J. Appl. Microbiol. 2009, 107, 65–71. [Google Scholar] [CrossRef]
- Polo, D.; Schaeffer, J.; Teunis, P.; Buchet, V.; Le Guyader, F.S. Infectivity and RNA Persistence of a Norovirus Surrogate, the Tulane Virus, in Oysters. Front. Microbiol. 2018, 9, 716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cromeans, T.; Park, G.W.; Costantini, V.; Lee, D.; Wang, Q.; Farkas, T.; Lee, A.; Vinjé, J. Comprehensive Comparison of Cultivable Norovirus Surrogates in Response to Different Inactivation and Disinfection Treatments. Appl. Environ. Microbiol. 2014, 80, 5743–5751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, G.; Linden, K.G.; Sobsey, M. Inactivation of murine norovirus, feline calicivirus and echovirus 12 as surrogates for human norovirus (NoV) and coliphage (F+) MS2 by ultraviolet light (254 nm) and the effect of cell association on UV inactivation. Lett. Appl. Microbiol. 2011, 52, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Watson, K.A.; Selinka, H.-C.; Carthy, C.M.; Klingel, K.; McManus, B.M.; Kandolf, R. Cleavage of RasGAP and Phosphorylation of Mitogen-Activated Protein Kinase in the Course of Coxsackievirus B3 Replication. J. Virol. 1999, 73, 3587–3594. [Google Scholar] [CrossRef] [Green Version]
- Hammon, W.M.; Ludwig, E.H.; Pavia, R.A.; McCloskey, L.W.; Sather, G.E. Problems Raised by Certain ECHO Viruses in the Attempted Laboratory Detection of Poliomyelitis Virus Infection. Ann. N. Y. Acad. Sci. 1957, 67, 304–310. [Google Scholar] [CrossRef]
- Dawson, D.; Paish, A.; Staffell, L.; Seymour, I.; Appleton, H. Survival of viruses on fresh produce, using MS2 as a surrogate for norovirus. J. Appl. Microbiol. 2005, 98, 203–209. [Google Scholar] [CrossRef]
- Tung-Thompson, G.; Libera, D.A.; Koch, K.L.; de los Reyes, F.L., III; Jaykus, L.-A. Aerosolization of a Human Norovirus Surrogate, Bacteriophage MS2, during Simulated Vomiting. PLoS ONE 2015, 10, e0134277. [Google Scholar] [CrossRef]
- Seo, K.; Lee, J.E.; Lim, M.Y.; Ko, G. Effect of Temperature, pH, and NaCl on the Inactivation Kinetics of Murine Norovirus. J. Food Prot. 2012, 75, 533–540. [Google Scholar] [CrossRef]
- Doultree, J.; Druce, J.; Birch, C.; Bowden, D.; Marshall, J. Inactivation of feline calicivirus, a Norwalk virus surrogate. J. Hosp. Infect. 1999, 41, 51–57. [Google Scholar] [CrossRef]
- Green, K. Human Caliciviruses. Fields Virol. 2001, 1, 841–874. [Google Scholar]
- Karst, S.M.; Wobus, C.E.; Lay, M.; Davidson, J.; Virgin, H.W. STAT1-Dependent Innate Immunity to a Norwalk-Like Virus. Science 2003, 299, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.L.; Papafragkou, E.; Park, G.W.; Osborne, J.; Jaykus, L.-A.; Vinjé, J. Surrogates for the Study of Norovirus Stability and Inactivation in the Environment: A Comparison of Murine Norovirus and Feline Calicivirus. J. Food Prot. 2006, 69, 2761–2765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drouaz, N.; Schaeffer, J.; Farkas, T.; Le Pendu, J.; Le Guyader, F.S. Tulane Virus as a Potential Surrogate to Mimic Norovirus Behavior in Oysters. Appl. Environ. Microbiol. 2015, 81, 5249–5256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkas, T.; Sestak, K.; Wei, C.; Jiang, X. Characterization of a Rhesus Monkey Calicivirus Representing a New Genus of Caliciviridae. J. Virol. 2008, 82, 5408–5416. [Google Scholar] [CrossRef] [Green Version]
- Park, B.-J.; Ahn, H.-S.; Han, S.-H.; Go, H.-J.; Lyoo, E.-L.; Choi, C.; Myoung, J.; Lee, J.-B.; Park, S.-Y.; Song, C.-S.; et al. Coding-Complete Genome Sequence of a Recombinant Human Norovirus Strain Identified as Subtype GII.p12_GII.3. Microbiol. Resour. Announc. 2020, 9. [Google Scholar] [CrossRef] [Green Version]
- Le Guyader, F.S.; Maalouf, H.; Le Pendu, J. Oysters and norovirus: Something special? Virologie 2013, 17, 253–263. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, A.-N.; Lee, K.-H.; Ha, S.-D. Ultraviolet-C efficacy against a norovirus surrogate and hepatitis A virus on a stainless steel surface. Int. J. Food Microbiol. 2015, 211, 73–78. [Google Scholar] [CrossRef]
- Lee, J.; Zoh, K.; Ko, G. Inactivation and UV Disinfection of Murine Norovirus with TiO2 under Various Environmental Conditions. Appl. Environ. Microbiol. 2008, 74, 2111–2117. [Google Scholar] [CrossRef] [Green Version]
- Vimont, A.; Fliss, I.; Jean, J.; Deng, Y.; Liu, X.; Wu, J.; Lee, J.; Chen, S.; Cheng, Y.; Zhang, C.; et al. Efficacy and Mechanisms of Murine Norovirus Inhibition by Pulsed-Light Technology. Appl. Environ. Microbiol. 2015, 81, 2950–2957. [Google Scholar] [CrossRef] [Green Version]
- Raeiszadeh, M.; Adeli, B. A Critical Review on Ultraviolet Disinfection Systems against COVID-19 Outbreak: Applicability, Validation, and Safety Considerations. ACS Photon. 2020, 7, 2941–2951. [Google Scholar] [CrossRef]
- Mariita, R.M.; Miller, A.C.W.; Randive, R.V. Evaluation of the virucidal efficacy of Klaran UVC LEDs against surface-dried norovirus. Access Microbiol. 2021, 4, 000323. [Google Scholar] [CrossRef]
- Mariita, R.M.; Randive, R.V. Disinfection of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium and Acinetobacter baumannii using Klaran WD array system. Access Microbiol. 2021, 3, 000194. [Google Scholar] [CrossRef] [PubMed]
- Mariita, R.M.; Davis, J.H.; Lottridge, M.M.; Randive, R.V. Shining light on multi-drug resistant Candida auris: Ultraviolet-C disinfection, wavelength sensitivity, and prevention of biofilm formation of an emerging yeast pathogen. MicrobiologyOpen 2022, 11, e1261. [Google Scholar] [CrossRef]
- Bolton, J.R.; Cotton, C.A. The Ultraviolet Disinfection Handbook; American Water Works Association: Denver, CO, USA, 2011; ISBN 1-61300-077-4. [Google Scholar]
- Jacques, S. On Optical Irradiance versus Fluence Rate. Available online: https://omlc.org/news/sep05/irradiancemovie.html (accessed on 25 October 2021).
- Rahn, R.O. Potassium Iodide as a Chemical Actinometer for 254 nm Radiation: Use of lodate as an Electron Scavenger. Photochem. Photobiol. 1997, 66, 450–455. [Google Scholar] [CrossRef]
- Rahn, R.O.; Bolton, J.; Stefan, M.I. The Iodide/Iodate Actinometer in UV Disinfection: Determination of the Fluence Rate Distribution in UV Reactors. Photochem. Photobiol. 2006, 82, 611–615. [Google Scholar] [CrossRef]
- UVC Dosimeter Verifies UVC Exposure. Available online: https://www.americanultraviolet.com/germicidal-healthcare-solutions/uvc-dosimeter.html (accessed on 22 November 2021).
- Bondokov, R.T.; Branagan, S.P.; Ishigami, N.; Grandusky, J.; Nagatomi, T.; Tatsuta, K.; Miebach, T.; Chen, J. Two-Inch Aluminum Nitride (AIN) Single Crystal Growth for Commercial Applications. ECS Trans. 2021, 104, 37–48. [Google Scholar] [CrossRef]
- Mariita, R.M.; Wilson Miller, A.C.; Randive, R.V.; McKay, L.G.A.; Storm, N.; Griffiths, A. Disinfection of SARS-CoV-2 Using UVC Reveals Wavelength Sensitivity Contributes towards Rapid Virucidal Activity. medRxiv 2021, 1–13. Available online: https://www.medrxiv.org/content/10.1101/2021.06.30.21259769v2 (accessed on 29 November 2021).
- Mariita, R.M.; Peterson, J.W. Not All Wavelengths Are Created Equal: Disinfection of SARS-CoV-2 Using UVC Radiation Is Wavelength-Dependent. Access Microbiol. 2021, 3, 276. [Google Scholar] [CrossRef]
- Minamikawa, T.; Koma, T.; Suzuki, A.; Mizuno, T.; Nagamatsu, K.; Arimochi, H.; Tsuchiya, K.; Matsuoka, K.; Yasui, T.; Yasutomo, K.; et al. Quantitative evaluation of SARS-CoV-2 inactivation using a deep ultraviolet light-emitting diode. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- De Abajo, F.J.G.; Hernández, R.J.; Kaminer, I.; Meyerhans, A.; Rosell-Llompart, J.; Sanchez-Elsner, T. Back to Normal: An Old Physics Route to Reduce SARS-CoV-2 Transmission in Indoor Spaces. ACS Nano 2020, 14, 7704–7713. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Li, C.-S. Inactivation of Viruses on Surfaces by Ultraviolet Germicidal Irradiation. J. Occup. Environ. Hyg. 2007, 4, 400–405. [Google Scholar] [CrossRef]
- Gallardo-Flores, C.; Colpitts, C. Cyclophilins and Their Roles in Hepatitis C Virus and Flavivirus Infections: Perspectives for Novel Antiviral Approaches. Pathogens 2021, 10, 902. [Google Scholar] [CrossRef] [PubMed]
- Najm, I. An alternative interpretation of disinfection kinetics. J. Am. Water Work. Assoc. 2006, 98, 93–101. [Google Scholar] [CrossRef]
- Hong, H.; Shin, W.; Oh, J.; Lee, S.; Kim, T.; Lee, W.; Choi, J.; Suh, S.; Kim, K. Standard for the Quantification of a Sterilization Effect Using an Artificial Intelligence Disinfection Robot. Sensors 2021, 21, 7776. [Google Scholar] [CrossRef] [PubMed]
- NEA Advises the Public Against Purchasing Unsafe Ultraviolet-C Sterilisers for Home Use. Available online: https://www.nea.gov.sg/media/news/advisories/index/nea-advises-the-public-against-purchasing-unsafe-ultraviolet-c-sterilisers-for-home-use (accessed on 29 November 2021).
- Risk of Exposure to Unsafe Levels of Radiation with Safe-T-Lite UV WAND: FDA Safety Communication. Available online: https://www.nea.gov.sg/media/news/advisories/index/nea-advises-the-public-against-purchasing-unsafe-ultraviolet-c-sterilisers-for-home-use (accessed on 29 November 2021).
- Consumer Council. Consumers Reminded of Broad Limitations on Portable UV Disinfection Devices Do Not Expose Skin and Eyes to UVC to Avoid Injuries. Available online: https://www.consumer.org.hk/en/press-release/532-portable-uv-disinfection-devices (accessed on 29 November 2021).
- Kheyrandish, A.; Mohseni, M.; Taghipour, F. Protocol to Determine Radiant Power Output and Fluence in UV LED Systems. 2019. Available online: https://uvsolutionsmag.com/articles/2019/protocol-to-determine-radiant-power-output-and-fluence-in-uv-led-systems/ (accessed on 29 November 2021).
- Bolton, J.R.; Mayor-Smith, I.; Linden, K.G. Rethinking the Concepts of Fluence (UV Dose) and Fluence Rate: The Importance of Photon-based Units—A Systemic Review. Photochem. Photobiol. 2015, 91, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.H.; Suwan, P.; Koottatep, T.; Beck, S.E. Application of a novel, continuous-feeding ultraviolet light emitting diode (UV-LED) system to disinfect domestic wastewater for discharge or agricultural reuse. Water Res. 2019, 153, 53–62. [Google Scholar] [CrossRef]
- Gullian, M.; Espinosa-Faller, F.J.; Núñez, A.; López-Barahona, N. Effect of turbidity on the ultraviolet disinfection performance in recirculating aquaculture systems with low water exchange. Aquac. Res. 2011, 43, 595–606. [Google Scholar] [CrossRef]
- Sato, S.; Matsumoto, N.; Hisaie, K.; Uematsu, S. Alcohol abrogates human norovirus infectivity in a pH-dependent manner. Sci. Rep. 2020, 10, 15878. [Google Scholar] [CrossRef]
- Casas-Monroy, O.; Linley, R.D.; Chan, P.-S.; Kydd, J.; Byllaardt, J.V.; Bailey, S. Evaluating efficacy of filtration + UV-C radiation for ballast water treatment at different temperatures. J. Sea Res. 2018, 133, 20–28. [Google Scholar] [CrossRef]
- Cheng, Z.; Ling, L.; Shang, C. Near-Ultraviolet Light-Driven Photocatalytic Chlorine Activation Process with Novel Chlorine Activation Mechanisms. ACS EST Water 2021, 1, 2067–2075. [Google Scholar] [CrossRef]
- Lin, Q.; Lim, J.Y.C.; Xue, K.; Yew, P.Y.M.; Owh, C.; Chee, P.L.; Loh, X.J. Sanitizing agents for virus inactivation and disinfection. VIEW 2020, 1, e16. [Google Scholar] [CrossRef]
- Jubinville, E.; Girard, M.; Trudel-Ferland, M.; Fliss, I.; Jean, J. Inactivation of Murine Norovirus Suspended in Organic Matter Simulating Actual Conditions of Viral Contamination. Food Environ. Virol. 2021, 13, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, S.; Sealey, L.; Patterson, J.; Odegaard, C. Not All Water Is Created Equal: The Effects of Water Characteristics on MS2 Stability and Dose Response. Available online: https://www.iuva.org/resources/2018_IUVA_Americas_Conference/Technical-Proceedings/Validation%20and%20Lab%20Approaches/Verhoeven_Shawn-Not-All-Water-Is-Created-Equal.pdf (accessed on 22 November 2021).
- Patra, V.; Byrne, S.N.; Wolf, P. The Skin Microbiome: Is It Affected by UV-induced Immune Suppression? Front. Microbiol. 2016, 7, 1235. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Petersen, F.C.; Shekhar, S. Commensal bacteria: An emerging player in defense against respiratory pathogens. Front. Immunol. 2019, 10, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Ma, W.-T.; Pang, M.; Fan, Q.-L.; Hua, J.-L. The Commensal Microbiota and Viral Infection: A Comprehensive Review. Front. Immunol. 2019, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Flowers, L.; Grice, E.A. The Skin Microbiota: Balancing Risk and Reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef]
- Pullerits, K.; Ahlinder, J.; Holmer, L.; Salomonsson, E.; Öhrman, C.; Jacobsson, K.; Dryselius, R.; Forsman, M.; Paul, C.J.; Rådström, P. Impact of UV irradiation at full scale on bacterial communities in drinking water. npj Clean Water 2020, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Hannigan, G.D.; Meisel, J.S.; Tyldsley, A.S.; Zheng, Q.; Hodkinson, B.P.; SanMiguel, A.J.; Minot, S.; Bushman, F.D.; Grice, E.A. The Human Skin Double-Stranded DNA Virome: Topographical and Temporal Diversity, Genetic Enrichment, and Dynamic Associations with the Host Microbiome. mBio 2015, 6, e01578-15. [Google Scholar] [CrossRef] [Green Version]
- Fukui, T.; Niikura, T.; Oda, T.; Kumabe, Y.; Ohashi, H.; Sasaki, M.; Igarashi, T.; Kunisada, M.; Yamano, N.; Oe, K.; et al. Exploratory clinical trial on the safety and bactericidal effect of 222-nm ultraviolet C irradiation in healthy humans. PLoS ONE 2020, 15, e0235948. [Google Scholar] [CrossRef]
- Yamano, N.; Kunisada, M.; Kaidzu, S.; Sugihara, K.; Nishiaki-Sawada, A.; Ohashi, H.; Yoshioka, A.; Igarashi, T.; Ohira, A.; Tanito, M.; et al. Long-term Effects of 222-nm ultraviolet radiation C Sterilizing Lamps on Mice Susceptible to Ultraviolet Radiation. Photochem. Photobiol. 2020, 96, 853–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaidzu, S.; Sugihara, K.; Sasaki, M.; Nishiaki, A.; Igarashi, T.; Tanito, M. Evaluation of acute corneal damage induced by 222-nm and 254-nm ultraviolet light in Sprague–Dawley rats. Free Radic. Res. 2019, 53, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Kaidzu, S.; Sugihara, K.; Sasaki, M.; Nishiaki, A.; Ohashi, H.; Igarashi, T.; Tanito, M. Re-Evaluation of Rat Corneal Damage by Short-Wavelength UV Revealed Extremely Less Hazardous Property of Far-UV-C. Photochem. Photobiol. 2021, 97, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Sliney, D.H.; Stuck, B.E. A Need to Revise Human Exposure Limits for Ultraviolet UV-C Radiation. Photochem. Photobiol. 2021, 97, 485–492. [Google Scholar] [CrossRef]
- Tett, A.; Pasolli, E.; Farina, S.; Truong, D.T.; Asnicar, F.; Zolfo, M.; Beghini, F.; Armanini, F.; Jousson, O.; De Sanctis, V.; et al. Unexplored diversity and strain-level structure of the skin microbiome associated with psoriasis. npj Biofilms Microbiomes 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Neu, D.T.; Mead, K.R.; McClelland, T.L.; Lindsley, W.G.; Martin, S.B.; Heil, G.; See, M.; Feng, H.A. Surface Dosimetry of Ultraviolet Germicidal Irradiation Using a Colorimetric Technique. Ann. Work Expo. Health 2021, 65, 605–611. [Google Scholar] [CrossRef]
- Mamahlodi, M.T. Potential benefits and harms of the use of UV radiation in transmission of tuberculosis in South African health facilities. J. Public Health Afr. 2019, 10, 742. [Google Scholar] [CrossRef]
- Moon, Y.; Han, S.; Son, J.W.; Park, S.H.; Ha, S.-D. Impact of ultraviolet-C and peroxyacetic acid against murine norovirus on stainless steel and lettuce. Food Control 2021, 130, 108378. [Google Scholar] [CrossRef]
- Jean, J.; Morales-Rayas, R.; Anoman, M.-N.; Lamhoujeb, S. Inactivation of hepatitis A virus and norovirus surrogate in suspension and on food-contact surfaces using pulsed UV light (pulsed light inactivation of food-borne viruses). Food Microbiol. 2011, 28, 568–572. [Google Scholar] [CrossRef]
- Li, D.; Baert, L.; De Jonghe, M.; Van Coillie, E.; Ryckeboer, J.; Devlieghere, F.; Uyttendaele, M. Inactivation of Murine Norovirus 1, Coliphage φX174, and Bacillus fragilis Phage B40-8 on Surfaces and Fresh-Cut Iceberg Lettuce by Hydrogen Peroxide and UV Light. Appl. Environ. Microbiol. 2011, 77, 1399–1404. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Solà, J.; Abadias, I.; Colàs-Medà, P.; Anguera, M.; Viñas, I. Inactivation of Salmonella enterica, Listeria monocytogenes and murine norovirus (MNV-1) on fresh strawberries by conventional and water-assisted ultraviolet light (UV-C). Postharvest Biol. Technol. 2021, 174, 111447. [Google Scholar] [CrossRef]
- Kato, T.; Tohma, H.; Miki, O.; Shibata, T.; Tamura, M. Degradation of Norovirus in Sewage Treatment Water by Photocatalytic Ultraviolet Disinfection. Nippon. Steel Tech. Rep. 2005, 92, 41–44. [Google Scholar]
- Barrett, M.; Fitzhenry, K.; O’Flaherty, V.; Dore, W.; Keaveney, S.; Cormican, M.; Rowan, N.; Clifford, E. Detection, fate and inactivation of pathogenic norovirus employing settlement and UV treatment in wastewater treatment facilities. Sci. Total Environ. 2016, 568, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.A.T.; Nascimento, M.A.; Barardi, C.R.M. Effect of UV Light on the Inactivation of Recombinant Human Adenovirus and Murine Norovirus Seeded in Seawater in Shellfish Depuration Tanks. Food Environ. Virol. 2015, 7, 67–75. [Google Scholar] [CrossRef]
- Watts, M.J.; Linden, K.G. Chlorine photolysis and subsequent OH radical production during UV treatment of chlorinated water. Water Res. 2007, 41, 2871–2878. [Google Scholar] [CrossRef]
- Julius, A.A.; Sawyer, S.M. Control Systems Challenges in Energy Efficient Portable UV Based Water Sterilizer. In Proceedings of the 2010 American Control Conference, Baltimore, MD, USA, 30 July 2010; pp. 3617–3622. [Google Scholar]
- Alcala, J.R.; Gratton, E.; Prendergast, F.G. Fluorescence lifetime distributions in proteins. Biophys. J. 1987, 51, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, A.; Vernon, M.; Cormier, J.-F.; Beaulieu, R.; Mafu, A.A.; Fréchette, J.; Vallée, R. Optical characterization of Pseudomonas fluorescens on meat surfaces using time-resolved fluorescence. J. Biomed. Opt. 2006, 11, 1–7. [Google Scholar] [CrossRef]
- Whitham, T.; Saba, N.; Woo, H.; Carlson, K.; Bokzek, L.; Ryu, H. Efficacy of Inactivation of Pseudomonas aeruginosa by Multiple-Wavelength UV LEDs. Available online: https://cfpub.epa.gov/si/si_public_file_download.cfm?p_download_id=536619&Lab=NRMRL (accessed on 29 November 2021).
- ASHRAE Ultraviolet Lamp Systems. Available online: https://www.ashrae.org/file%20library/technical%20resources/covid-19/i-p_s16_ch17.pdf (accessed on 29 November 2021).
- Shimoda, H.; Matsuda, J.; Iwasaki, T.; Hayasaka, D. Efficacy of 265-nm ultraviolet light in inactivating infectious SARS-CoV-2. J. Photochem. Photobiol. 2021, 7, 100050. [Google Scholar] [CrossRef]

| Surrogate | Advantages | Disadvantages | Source |
|---|---|---|---|
| Feline calicivirus (FCV) |
|
| [49,65,66,67,68,69,70,71,72,73,74] |
| Murine norovirus (MN) |
|
| [57,65,66,68,69,70,75,76] |
| Tulane virus (TV) |
|
| [21,69,77,78] |
| Echovirus 12 |
|
| [79,80,81] |
| MS2 and Qβ |
|
| [71,82,83,84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariita, R.M.; Davis, J.H.; Randive, R.V. Illuminating Human Norovirus: A Perspective on Disinfection of Water and Surfaces Using UVC, Norovirus Model Organisms, and Radiation Safety Considerations. Pathogens 2022, 11, 226. https://doi.org/10.3390/pathogens11020226
Mariita RM, Davis JH, Randive RV. Illuminating Human Norovirus: A Perspective on Disinfection of Water and Surfaces Using UVC, Norovirus Model Organisms, and Radiation Safety Considerations. Pathogens. 2022; 11(2):226. https://doi.org/10.3390/pathogens11020226
Chicago/Turabian StyleMariita, Richard M., James H. Davis, and Rajul V. Randive. 2022. "Illuminating Human Norovirus: A Perspective on Disinfection of Water and Surfaces Using UVC, Norovirus Model Organisms, and Radiation Safety Considerations" Pathogens 11, no. 2: 226. https://doi.org/10.3390/pathogens11020226