Potential for Exposure to Particles and Gases throughout Vat Photopolymerization Additive Manufacturing Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bulk Resin Characterization
2.2. Test Chambers
2.3. Air Monitoring
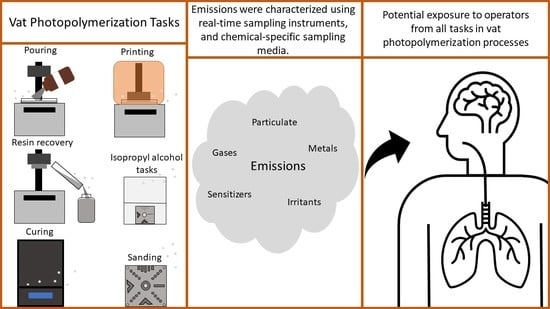
2.4. Task Descriptions
- Pouring: Before printing, the resin bottle was mixed thoroughly by shaking vigorously. The bottle was uncapped, the printer cover was opened, and the resin was poured into the vat until the level reached the manufacturer-specified maximum fill line. Immediately after pouring, the printer cover was closed, and the bottle was recapped and removed from the chamber.
- Printing: An artifact from the National Institute of Standards and Technology (NIST) was printed at a 50% scale of the full size [31]. The layer height of the NIST file for the SLA printer was 0.05 mm (PreForm v2.19.3, Formlabs Inc.) and 0.015 mm for the DLP printer (XMaker v2.5.4, Makex Co., Ltd.), which were the software recommended settings for each printer. Supports for the part were automatically generated in each software. The specific software meant for this DLP printer required manual input of four specific exposure times (raft [120 s], base [40 s], model [20 s], and darktime [2 s]). The raft is the layer that adheres the part to the build plate; the base is the layers of support between the raft and the model; the model is the remaining layers of the print job. Darktime refers to the build plate being raised and lowered after the completion of each layer. These exposure times were chosen based on the manufacturer’s recommended settings as well as trial and error. The lid on both printers must be closed in order for the printer to operate
- Recovery: After printing, any unused resin was recovered from the vat from each machine and saved for reuse. To perform this post-printing task, the printer cover was opened, and the vat containing unused resin was removed from the printer. The resin was slowly poured into a 250 mL polypropylene bottle that was wrapped in foil to prevent exposure to light, and using a putty knife provided by the manufacturer, the vat was gently scraped to remove as much resin as possible. The printer cover was closed and remained in the chamber, and vat and the resealed bottle that contained recovered resin were immediately removed from the chamber and stored in the dark. The printed part remained attached to the build plate, and a paper towel replaced the vat to prevent any interior damage from resin dripping.
- Rinsing: Built objects were subjected to sequential post-processing tasks. The first step was an IPA rinse. According to the printer manufacturers, for the SLA machine, the rinse task uses two containers whereas, for the DLP machine, the rinse task uses one container. For consistency, one container was used for both the SLA and DLP machines. The printer cover was opened to remove the build plate so the printed part could be removed. The printer cover was then closed (without the build plate), and the printed object was placed in a container with 500 mL of IPA (99.9%, HPLC Grade, Thermo Fisher Scientific, Waltham, MA, USA), the lid sealed, and the container was shaken for 1 to 2 min.
- Soaking: After rinsing, objects sat in their sealed containers without agitation to soak in IPA.
- Drying: The object was removed from the IPA container and placed on paper towels with the lights turned off in the chamber to air dry overnight.
- Curing: To finish the hardening (curing) of any under- or un-cured resins on the objects, they were treated with ultraviolet (UV) radiation and heat [32]. A commercially available UV curing chamber (Form Cure, Formlabs Inc.) was used to cure all printed objects. Build supports on the printed objects were removed with flush cutters before placing the objects inside the curing chamber. Each printed object was subjected to UV light at 405 nm in a 60 °C air atmosphere.
- Sanding: Printed objects were sanded using a sequence of three different grit sizes to give the finished product a cleaner and more professional look. Coarse grit (220) sandpaper was used to sand the surface where the supports were connected. Medium grit (660) and fine grit (1000) sandpaper were used to finish the part until it was smooth to the touch. Sanding took one minute for each grit with 10-min intervals between sanding to allow for airborne contaminant concentrations in the chamber to decay.
2.5. Statistical Analysis
3. Results
3.1. Pouring
3.2. Printing
3.3. Recovery
3.4. Rinsing
3.5. Soaking
3.6. Drying
3.7. Curing
3.8. Sanding
3.9. Relationships among Tasks
4. Discussion
4.1. Pouring
4.2. Printing
4.3. Recovery
4.4. Rinsing
4.5. Soaking
4.6. Drying
4.7. Curing
4.8. Sanding
4.9. Relationships among Tasks
4.10. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISO/ASTM. 52900; Additive Manufacturing—General Principles—Terminology. ISO: Geneva, Switzerland, 2015.
- Chen, R.; Yin, H.; Cole, I.S.; Shen, S.; Zhou, X.; Wang, Y.; Tang, S. Exposure, assessment and health hazards of particulate matter in metal additive manufacturing: A review. Chemosphere 2020, 259, 127452. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, E.; Kondej, D.; Kowalska, J.; Szewczyńska, M. State of the art in additive manufacturing and its possible chemical and particle hazards-review. Indoor Air 2021, 31, 1733–1758. [Google Scholar] [CrossRef] [PubMed]
- Leso, V.; Ercolano, M.L.; Mazzotta, I.; Romano, M.; Cannavacciuolo, F.; Iavicoli, I. Three-dimensional (3D) printing: Implications for risk assessment and management in occupational settings. Ann. Work. Expo. Health 2021, 65, 617–634. [Google Scholar] [CrossRef]
- Min, K.; Li, Y.; Wang, D.; Chen, B.; Ma, M.; Hu, L.; Liu, Q.; Jiang, G. 3D printing-induced fine particle and volatile organic compound emission: An emerging health risk. Environ. Sci. Technol. Lett. 2021, 8, 616–625. [Google Scholar] [CrossRef]
- Stefaniak, A.B.; Du Preez, S.; Du Plessis, J.L. Additive manufacturing for occupational Hhgiene: A comprehensive review of processes, emissions, & exposures. J. Toxicol. Environ. Health B Crit. Rev. 2021, 24, 173–222. [Google Scholar]
- du Preez, S.; Johnson, A.R.; LeBouf, R.F.; Linde, S.J.L.; Stefaniak, A.B.; Du Plessis, J. Exposures during industrial 3-D printing and post-processing tasks. Rapid Prototyp. J. 2018, 24, 865–871. [Google Scholar] [CrossRef]
- Graff, P.; Ståhlbom, B.; Nordenberg, E.; Graichen, A.; Johansson, P.; Karlsson, H. Evaluating measuring techniques for occupational exposure during additive manufacturing of metals: A pilot study. J. Indust. Ecol. 2017, 21, S120–S129. [Google Scholar] [CrossRef]
- Eden, G.R.; Tinnerberg, H.; Rosell, L.; Moller, R.; Almstrand, A.C.; Bredberg, A. Exploring methods for surveillance of occupational exposure from additive manufacturing in four different industrial facilities. Ann. Work Expo. Health 2022, 66, 163–177. [Google Scholar] [CrossRef]
- Pagac, M.; Hajnys, J.; Ma, Q.P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A review of vat photopolymerization technology: Materials, applications, challenges, and future trends of 3d printing. Polymers 2021, 13, 598. [Google Scholar] [CrossRef]
- Short, D.B.; Volk, D.; Badger, P.D.; Melzer, J.; Salerno, P.; Sirinterlikci, A. 3D printing (rapid prototyping) photopolymers: An emerging source of antimony to the environment. 3D Print Addit. Manuf. 2014, 1, 24–33. [Google Scholar] [CrossRef]
- Creytens, K.; Gilissen, L.; Huygens, S.; Goossens, A. A new application for epoxy resins resulting in occupational allergic contact dermatitis: The three-dimensional printing industry. Contact Dermat. 2017, 77, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, I.; Tamura, A.; Matsumura, M.; Miura, H.; Yui, N. Sensitization potential of dental resins: 2-Hydroxyethyl methacrylate and its water-soluble oligomers have immunostimulatory effects. PLoS ONE 2013, 8, e82540. [Google Scholar] [CrossRef] [PubMed]
- Heratizadeh, A.; Werfel, T.; Schubert, S.; Geier, J. Contact sensitization in dental technicians with occupational contact dermatitis. Data of the Information Network of Departments of Dermatology (IVDK) 2001–2015. Contact Dermat. 2018, 78, 266–273. [Google Scholar] [CrossRef]
- Väisänen, A.; Alonen, L.; Ylönen, S.; Hyttinen, M. Organic compound and particle emissions of additive manufacturing with photopolymer resins and chemical outgassing of manufactured resin products. J. Toxicol. Environ. Health A 2022, 85, 198–216. [Google Scholar] [CrossRef] [PubMed]
- Väisänen, A.J.K.; Hyttinen, M.; Ylönen, S.; Alonen, L. Occupational exposure to gaseous and particulate contaminants originating from additive manufacturing of liquid, powdered, and filament plastic materials and related post-processes. J Occup. Environ. Hyg. 2019, 16, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Mousawi, A.A.; Poriel, C.; Dumur, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.P.; Lalevée, J. Zinc tetraphenylporphyrin as high performance visible light photoinitiator of cationic photosensitive resins for LED projector 3D printing applications. Macromolecules 2017, 50, 746–753. [Google Scholar] [CrossRef]
- Czech, Z.; Butwin, A.; Gåuch, U.; Kabatc, J. Influence of selected photoinitiators on important properties of photoreactive acrylic pressure-sensitive adhesives. J. Appl. Polym. Sci. 2012, 123, 118–123. [Google Scholar] [CrossRef]
- Jakubiak, J.; Rabek, J.F. Photoinitiators for visible light polymerization. Polimery 1999, 44, X1–X461. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Campolo, D.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper and iron complexes as visible-light-sensitive photoinitiators of polymerization. J. Polym. Sci. A Polym. Chem. 2015, 53, 2673–2684. [Google Scholar] [CrossRef]
- NIOSH. Method 7303: Elements by ICP (Hot Block/HCl/HNO3 Digestion). In NIOSH Manual of Analytical Methods, 4th ed.; U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2003; Publication No. 2003-145. [Google Scholar]
- EPA. Method 8270: Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS). SW-846: Test Methods for Evaluating Solid Waste: Physical/Chemical Methods. U.S. Environmental Protection Agency. Available online: https://www.epa.gov/hw-sw846 (accessed on 2 February 2021).
- Stefaniak, A.B.; Bowers, L.N.; Knepp, A.K.; Luxton, T.P.; Peloquin, D.M.; Baumann, E.J.; Ham, J.E.; Wells, J.R.; Johnson, A.R.; LeBouf, R.F.; et al. Particle and vapor emissions from vat polymerization desktop-scale 3-dimensional printers. J. Occup. Environ. Hyg. 2019, 16, 519–531. [Google Scholar] [CrossRef]
- Hayes, A.C.; Osio-Norgaard, J.; Miller, S.; Whiting, G.L.; Vance, M.E. Air pollutant emissions from multi jet fusion, material-jetting, and digital light synthesis commercial 3D printers in a service bureau. Build. Environ. 2021, 202, 108008. [Google Scholar] [CrossRef]
- Zisook, R.E.; Simmons, B.D.; Vater, M.; Perez, A.; Donovan, E.P.; Paustenbach, D.J.; Cyrs, W.D. Emissions associated with operations of four different additive manufacturing or 3D printing technologies. J. Occup. Environ. Hyg. 2020, 17, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, M.D. Influence of technological parameters on the emision on DLP 3D printing process. Rev. Chim. 2019, 70, 4387–4392. [Google Scholar]
- Stefaniak, A.B.; Bowers, L.N.; Knepp, A.K.; Virji, M.A.; Birch, E.M.; Ham, J.E.; Wells, J.R.; Qi, C.; Schwegler-Berry, D.; Friend, S.; et al. Three-dimensional printing with nano-enabled filaments releases polymer particles containing carbon nanotubes into air. Indoor Air 2018, 28, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, A.B.; LeBouf, R.F.; Yi, J.; Ham, J.E.; Nurkewicz, T.R.; Schwegler-Berry, D.E.; Chen, B.T.; Wells, J.R.; Duling, M.G.; Lawrence, R.B.; et al. Characterization of chemical contaminants generated by a desktop fused deposition modeling 3-dimensional printer. J. Occup. Environ. Hyg. 2017, 14, 540–550. [Google Scholar] [CrossRef]
- OSHA. PV2252: Hydroxyethyl Methacrylate. U.S. Department of Labor, Occupational Safety and Health Administration; OSHA Salt Lake Technical Center: Salt Lake City, UT, USA, 1989. [Google Scholar]
- NIOSH. Method 2537: Methyl and Ethyl Methacrylate. In NIOSH Manual of Analytical Methods, 4th ed.; U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2003; Publication No. 2003-145. [Google Scholar]
- Moylan, S.; Slotwinski, J.; Cooke, A.; Jurrens, K.; Donmez, M.A. An additive manufacturing test artifact. J. Res. Natl. Inst. Stand. Technol. 2014, 119, 429–459. [Google Scholar] [CrossRef]
- Endruweit, A.; Johnson, M.S.; Long, A.C. Curing of composite components by ultraviolet radiation: A review. Polym. Compos. 2006, 27, 119–128. [Google Scholar] [CrossRef]
- Ganser, G.H.; Hewett, P. An accurate substitution method for analyzing censored data. J. Occup. Environ. Hyg. 2010, 7, 233–244. [Google Scholar] [CrossRef]
- Zhang, Q.; Davis, A.Y.; Black, M.S. Emissions and chemical exposure potentials from stereolithography vat photopolymerization 3D printing and post-processing units. ACS Chem. Health Saf. 2022, 29, 184–191. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L. Total volatile organic compound emission evaluation and control for stereolithography additive manufacturing process. J. Clean. Prod. 2018, 170, 1268–1278. [Google Scholar] [CrossRef]
- Bressot, C.; Morgeneyer, M.; Aguerre-Chariol, O.; Bouillard, J.; Zaras, K.; Visser, G.W.; Meier, R.J. Sanding and analysis of dust from nano-silica filled composite resins for stereolithography. Chem. Eng. Res. Des. 2020, 156, 23–30. [Google Scholar] [CrossRef]
- Hashmi, A.W.; Mali, H.S.; Meena, A. The Surface Quality Improvement Methods for FDM Printed Parts: A Review. In Fused Deposition Modeling Based 3D Printing; Dave, H.K., Davim, J.P., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 167–194. [Google Scholar]
- NIOSH. Pocket Guide to Chemical Hazards. Available online: http://www.cdc.gov/niosh/npg/default.html (accessed on 20 April 2021).
- Formlabs. Safety Data Sheet: Grey Photoactive Resin, Version 1; Formlabs Inc.: Somerville, MA, USA, 2016. [Google Scholar]
- IARC. Re-Evaluation of Some Organic Chemicals, Hydrazine, and Hydrogen Peroxide; International Agency for Research on Cancer: Lyon, France, 1999. [Google Scholar]

| Task | SLA (minutes) | DLP (minutes) | Decay (minutes) |
|---|---|---|---|
| Pouring (resin) | 0.5–0.7 | 0.5–0.8 | 60 |
| Printing (resin) | 145 | 163 | 60 |
| Recovery (resin) | 1–2 | 1–2 | 60 |
| Rinsing (IPA) | 2 | 1 | n/a |
| Soaking (IPA) | 20 | 20 | n/a |
| Drying (IPA) | 90 | 90 | 90 |
| Curing (resin and IPA) | 44 | 44 | 180 |
| Sanding | 3 | 3 | 60 |
| Particles | Gases | ||||||
|---|---|---|---|---|---|---|---|
| CNC | FMPS | APS | PID | ||||
| Task | Process | Conc. (#/cm3) * | Conc. (#/cm3) * | Size, nm (GSD) ‡ | Conc. (#/cm3) * | Size, nm (GSD) ‡ | TVOC (µg/m3) * |
| Pouring (resin) | SLA | 44.0 (2.0) A,† | 14.1 (2.2) A,† | 51.4 (1.3) | 0.1 (2.9) B | 678.2 (1.3) | -- |
| DLP | -- | -- | 55.4 (1.3) | -- | 696.6 (1.4) | 1.1 (1.8) D | |
| Printing (resin) | SLA | -- | -- | 40.6 (1.5) | -- | 658.2 (1.2) | 61.0 (1.7) D |
| DLP | -- | -- | 33.3 (1.5) | -- | 654.4 (1.3) | -- | |
| Recovery (resin) | SLA | -- | -- | 41.0 (1.4) | 0.1 (1.9) B,⸸ | 801.0 (1.6) | -- |
| DLP | 7.9 (1.6) A | -- | 29.8 (1.5) | 0.2 (1.8) B,⸸ | 876.2 (1.6) | 0.4 (4.3) C,D,† | |
| Rinsing (IPA) | SLA | -- | 10.5 (3.5) A,& | 44.2 (1.5) | 0.1 (1.4) B,† | 779.8 (1.5) | 1042.5 (4.4) C |
| DLP | 8.8 (2.7) A | 31.7 (3.1) A,⸸ | 29.6 (1.6) | 0.3 (1.1) B,⸸ | 831.8 (1.6) | 0.4 (2.6) C,† | |
| Soaking (IPA) | SLA | 20.7 (1.7) A | -- | 48.6 (1.4) | 0.1 (1.8) B | 769.4 (1.5) | 8080.7 (1.9) A |
| DLP | 27.0 (1.9) A | -- | 33.1 (1.5) | 0.5 (3.0) B,⸸ | 764.4 (1.5) | 15,176.8 (1.6) A | |
| Drying (IPA) | SLA | -- | -- | 49.1 (1.5) | -- | 695.2 (1.3) | 8322.3 (1.5) A |
| DLP | -- | -- | 39.4 (1.6) | -- | 726.2 (1.4) | 13,384.9 (1.3) A | |
| Curing | SLA | n/a | -- | 36.2 (1.3) | n/a | n/a | 1867.2 (1.0) A,B |
| DLP | n/a | -- | 44.3 (1.4) | n/a | n/a | 1654.2 (1.1) A,B | |
| Sanding | SLA | -- | n/a | n/a | 4.6 (1.9) A,⸸ | 1334.8 (1.9) A | n/a |
| DLP | -- | n/a | n/a | 0.3 (4.7) A,⸸ | 1024.5 (1.7) B | n/a | |
| Particles | Gases | ||||
|---|---|---|---|---|---|
| CNC | FMPS | APS | PID | ||
| Task | Process | Conc. (#/cm3) * | Conc. (#/cm3) * | Conc. (#/cm3) ‡ | TVOC (µg/m3) ‡ |
| Pouring (resin) | SLA | 57.9 (2.3) B,C | 325.5 (2.9) B | 0.2 (2.2) | 3.6 (1.8) ⸸ |
| DLP | 39.3 (1.7) B,C | 197.9 (2.7) B | -- | 5.5 (1.2) | |
| Printing (resin) | SLA | 125.0 (1.4) A | 907.2 (1.4) A | 0.3 (1.1) | 140.7 (1.5) |
| DLP | 270.5 (1.5) A | 1085.2 (1.7) A | 2.7 (4.2) | 11.2 (1.4) | |
| Recovery (resin) | SLA | 35.2 (1.6) B,C | 456.8 (1.6) A,B,! | 0.1 (2.0) | 1.8 (2.0) |
| DLP | 52.2 (1.4) B,C | 790.3 (1.7) A,B | 0.4 (1.7) ⸸ | 4.3 (1.9) | |
| Rinsing (IPA) | SLA | 28.2 (1.4) C,⸸ | 528.8 (1.9) A,B,! | 0.2 (1.5) ⸸ | 9113.0 (2.9) |
| DLP | 38.9 (1.6) C | 685.1 (3.7) A,B | 0.7 (1.7) ⸸ | 6.0 (1.5) | |
| Soaking (IPA) | SLA | 80.4 (1.2) A,B | 554.4 (1.5) A,B,! | 0.3 (1.8) | 22,890.2 (2.2) |
| DLP | 97.9 (1.4) A,B | 623.5 (2.5) A,B | 1.1 (2.6) ⸸ | 36,790.9 (2.0) | |
| Drying (IPA) | SLA | 98.0 (1.5) A | 1206.0 (1.7) A,! | 0.3 (2.1) | 19,443.5 (1.4) |
| DLP | 197.6 (2.2) A | 1010.8 (1.3) A | 3.0 (6.1) | 33,417.3 (1.6) | |
| Curing | SLA | n/a | 730.8 (1.8) A | n/a | 3083.4 (1.0) |
| DLP | n/a | 869.6 (1.4) A | n/a | 2990.0 (1.0) | |
| Sanding | SLA | 57.3 (1.8) B,C | n/a | 8.3 (1.9) | n/a |
| DLP | 51.1 (3.0) B,C | n/a | 0.6 (4.3) ⸸ | n/a | |
| Task | Process 2 | 2-Hydroxypropyl Methacrylate * (µg/m3) | Acetaldehyde (µg/m3) | Acetone (µg/m3) | Ethanol ‡ (µg/m3) | Methylene Chloride (µg/m3) | Styrene (µg/m3) |
|---|---|---|---|---|---|---|---|
| Pouring (resin) | SLA | 50.7 | 14.4–18.2 † | 2.1–26.0 ⸸ | 3.6–14.0 ⸸ | 23.5–76.0 ⸸ | <1.52 |
| DLP | <43.4 | 18.6–20.6 | 21.1 (1.4) | 6.7–26.2 ⸸ | 6.2 (2.5) | 4.1–8.9 | |
| Printing (resin) | SLA | 18.1–21.9 | 10.0–16.3 | 2.7–10.1 † | 0.02–4.5 † | 0.03–16.0 ⸸ | <1.52 |
| DLP | 31.2 (1.5) A,B | 8.3 (1.4) | 13.6 (1.5) | 7.4 (3.2) | 3.4 (5.4) | 4.5 | |
| Recovery (resin) | SLA | 43.8 (1.2) A,† | 19.7–35.4 | 22.8 (1.9) | 14.2 (3.8) ⸸ | 0.69–38.6 ⸸ | <1.52 |
| DLP | 58.5 (2.8) A,† | 10.9 | 17.5 (1.5) | 9.7 (2.3) | 5.8 (1.9) | 17.6 | |
| Curing | SLA | 11.3 | 0.3–15.7 ⸸ | 15.9–23.6 | 4.6 (2.0) ⸸ | 3.9 | <1.52 |
| DLP | 25.9 (1.8) B | 4.5–9.0 | 2.8–11.1 † | 0.6 (9.4) † | <0.204 | 2.0–4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowers, L.N.; Stefaniak, A.B.; Knepp, A.K.; LeBouf, R.F.; Martin, S.B., Jr.; Ranpara, A.C.; Burns, D.A.; Virji, M.A. Potential for Exposure to Particles and Gases throughout Vat Photopolymerization Additive Manufacturing Processes. Buildings 2022, 12, 1222. https://doi.org/10.3390/buildings12081222
Bowers LN, Stefaniak AB, Knepp AK, LeBouf RF, Martin SB Jr., Ranpara AC, Burns DA, Virji MA. Potential for Exposure to Particles and Gases throughout Vat Photopolymerization Additive Manufacturing Processes. Buildings. 2022; 12(8):1222. https://doi.org/10.3390/buildings12081222
Chicago/Turabian StyleBowers, Lauren N., Aleksandr B. Stefaniak, Alycia K. Knepp, Ryan F. LeBouf, Stephen B. Martin, Jr., Anand C. Ranpara, Dru A. Burns, and M. Abbas Virji. 2022. "Potential for Exposure to Particles and Gases throughout Vat Photopolymerization Additive Manufacturing Processes" Buildings 12, no. 8: 1222. https://doi.org/10.3390/buildings12081222