Gas-Solid Reaction Route toward the Production of Intermetallics from Their Corresponding Oxide Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation for Kinetic Studies
2.2. Methods (Techniques and Procedures)
3. Results and Discussion
3.1. Fe-Mo-O System
3.1.1. Isothermal Reduction of Fe2MoO4
3.1.2. Nonisothermal Reduction of Fe2MoO4
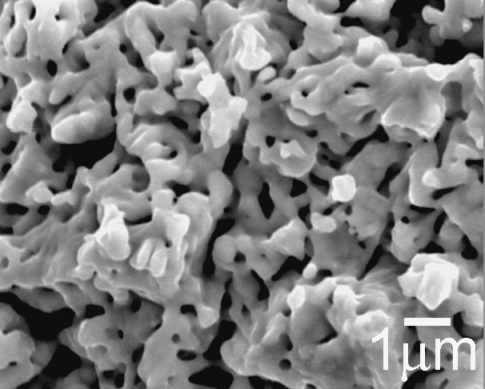
3.1.3. Characterization of Fe2Mo Intermetallic
3.1.4. Fluidized Bed Reduction of Fe2MoO4
3.2. Ni-W-O System
3.2.1. Reduction of Ni-W-O System
- The system is considered to be isothermal.
- The gas flow is plug flow.
- The mass transfer resistance for the reaction is small compared to the intrinsic reaction rate.
- The particle sizes are small enough that diffusive transport of gas through the product particles can be neglected.
3.2.2. Reduction-Carburization of Ni-W-O Mixed Oxides
3.2.3. Reduction-Nitridation of Ni-W-O Mixed Oxides
3.2.4. Thermal Diffusivity Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, M.; MacLeod, M.J.; Tessier, F.; DiSalvo, F.J. Mesoporous metal nitride materials prepared from bulk oxides. J. Am. Ceram. Soc. 2012, 95, 3084–3089. [Google Scholar] [CrossRef]
- Morales, R.; Sichen, D.; Seetharaman, S.; Arvanitidis, I. Reduction of Fe2MoO4 by hydrogen gas. Metall. Mater. Trans. B 2002, 33, 589–594. [Google Scholar] [CrossRef]
- Ahmed, H.M.; El-Geassy, A.A.; Viswanathan, N.N.; Seetharaman, S. Kinetics and Mathematical Modeling of Hydrogen Reduction of NiO-WO3 Precursors in Fluidized Bed Reactor. ISIJ Int. 2011, 51, 1383–1391. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Seetharaman, S. Isothermal dynamic thermal diffusivity studies of the reduction of NiO and NiWO4 precursors by hydrogen. Int. J. Mater. Res. 2011, 102, 1336–1344. [Google Scholar] [CrossRef]
- Heijwegen, C.; Rieck, G. Determination of the phase diagram of the Mo-Fe system using diffusion couples. J. Less Common Met. 1974, 37, 115–121. [Google Scholar] [CrossRef]
- Kleykamp, H.; Schauer, V. Phase equilibria and thermodynamics in the Fe-Mo and Fe-Mo-O systems. J. Less Common Met. 1981, 81, 229–238. [Google Scholar] [CrossRef]
- Rawlings, R.; Newey, C. Study of the Iron-Molybdenum System by Means of Diffusion Couples. J. Iron Steel Inst. 1968, 206, 723. [Google Scholar]
- Morales, R.; Arvanitidis, I.; Seetharaman, S. Interinsic Reduction of MoO3 by Hydrogen. Z. Metall. 2000, 91, 589. [Google Scholar]
- Morales, R.; Seetharaman, S.; Agarwala, V. Mechanical and structural characterization of uniaxially cold-pressed Fe2Mo powders. J. Mater. Res. 2002, 17, 1954–1959. [Google Scholar] [CrossRef]
- Ahmed, H.M.; El-Geassy, A.A.; Seetharaman, S. Kinetics of Reduction of NiO-WO3 Mixtures by Hydrogen. Metall. Mater. Trans. B 2010, 41, 161–172. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Seetharaman, S. Reduction-carburization of NiO-WO3 under isothermal conditions using H2-CH4 gas mixture. Metall. Mater. Trans. B 2010, 41, 173–181. [Google Scholar] [CrossRef]
- Bondarenko, V.; Pavlotskaya, E. High-temperature synthesis of tungsten carbide in a methane-hydrogen gas medium. Powder Metall. Met. Ceram. 1996, 34, 508–512. [Google Scholar] [CrossRef]
- Gao, L.; Kear, B. Synthesis of nanophase WC powder by a displacement reaction process. Nanostructured Mater. 1997, 9, 205–208. [Google Scholar] [CrossRef]
- Lebukhova, N.; Karpovich, N. Carbothermic reduction of cobalt and nickel tungstates. Inorg. Mater. 2006, 42, 310–315. [Google Scholar] [CrossRef]
- El-Geassy, A.; Nassir, N.A.; Ahmed, H.; Seetharaman, S. Simultaneous reduction nitridation for the synthesis of tungsten nitrides from Ni-W-O precursors. Powder Metall. 2013, 56, 411–419. [Google Scholar] [CrossRef]
- Taylor, R. Construction of apparatus for heat pulse thermal diffusivity measurements from 300–3000K. J. Phys. E Sci. Instrum. 1980, 13, 1193. [Google Scholar] [CrossRef]
- Sun, S.; Lu, W.K. Mathematical Modelling of Reactions in Iron Ore/Coal Composites. ISIJ Int. 1993, 33, 1062–1069. [Google Scholar] [CrossRef]
- Tsotsas, E.; Martin, H. Thermal conductivity of packed beds: A review. Chem. Eng. Process. Process Intensif. 1987, 22, 19–37. [Google Scholar] [CrossRef]
- Toth, I.; Lockington, N. The kinetics of metallic activation sintering of tungsten. J. Less Common Met. 1967, 12, 353–365. [Google Scholar] [CrossRef]
- Liu, L.; Loh, N.; Tay, B.; Tor, S.; Murakoshi, Y.; Maeda, R. Micro powder injection molding: Sintering kinetics of microstructured components. Scr. Mater. 2006, 55, 1103–1106. [Google Scholar] [CrossRef]
- Dominguez, O.; Bigot, J. Material transport mechanisms and activation energy in nanometric Fe powders based on sintering experiments. Nanostructured Mater. 1995, 6, 877–880. [Google Scholar] [CrossRef]
- Paul, B.; Jain, D.; Bidaye, A.; Sharma, I.; Pillai, C. Sintering kinetics of submicron sized cobalt powder. Thermochim. Acta 2009, 488, 54–59. [Google Scholar] [CrossRef]




















| Compound | Purity % | Supplier |
|---|---|---|
| MoO3 | 99.95 | Alfa Aesar; Karlsruhe, Germany |
| Fe2O3 | 99.8 | Alfa Aesar; Karlsruhe, Germany |
| Fe | 99.95 | Merck; Darmstadt, Germany |
| Fe | 98 | Merck; Darmstadt, Germany |
| NiO | 99 | Sigma-Aldrich (St. Louis, MO, USA) |
| WO3 | 99.9 | Atlantic Equipment Engineering (AEE) (Bergenfield, NJ, USA) |
| NiWO4 | 99 | Johnson Matthey Inc. (London, UK) |
| Iron with 98 pct was used for the fluidized bed experiments | ||
| Temp., K | Computed values | Experimental values * | ||
|---|---|---|---|---|
| 2nd stage | 3rd stage | 2nd stage | 3rd stage | |
| 973 | 1.81 × 10−3 | 0.60 × 10−3 | 1.09 × 10−3 | 0.60 × 10−3 |
| 1048 | 2.26 × 10−3 | 0.87 × 10−3 | 2.17 × 10−3 | 0.88 × 10−3 |
| 1123 | 2.67 × 10−3 | 1.14 × 10−3 | 2.56 × 10−3 | 1.09 × 10−3 |
| 1198 | 3.00 × 10−3 | 1.43 × 10−3 | 3.06 × 10−3 | 1.49 × 10−3 |
| 1273 | 3.28 × 10−3 | 1.72 × 10−3 | 3.34 × 10−3 | 1.72 × 10−3 |
| (Ni/Ni+W) molar ratio | Activation energy kJ/mol | ||
|---|---|---|---|
| 1st stage * | 2nd stage | 3rd stage | |
| TGA experiments | |||
| 0.7 | 17.9 | 62 | 51 |
| 0.6 | 17.5 | 51 | 43.9 |
| 0.5 | 18 | 37.9 | 35.5 |
| 0.46 | 20.6 | 38.2 | 34.5 |
| 0.4 | 40.3 ** | ||
| Fluidized bed experiments *** | |||
| 0.7 | --- | 58.6 | 50.8 |
| 0.5 | --- | 36.3 | 35 |
| 0.4 | --- | 46 ** | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, H.; Morales-Estrella, R.; Viswanathan, N.; Seetharaman, S. Gas-Solid Reaction Route toward the Production of Intermetallics from Their Corresponding Oxide Mixtures. Metals 2016, 6, 190. https://doi.org/10.3390/met6080190
Ahmed H, Morales-Estrella R, Viswanathan N, Seetharaman S. Gas-Solid Reaction Route toward the Production of Intermetallics from Their Corresponding Oxide Mixtures. Metals. 2016; 6(8):190. https://doi.org/10.3390/met6080190
Chicago/Turabian StyleAhmed, Hesham, R. Morales-Estrella, Nurin Viswanathan, and Seshadri Seetharaman. 2016. "Gas-Solid Reaction Route toward the Production of Intermetallics from Their Corresponding Oxide Mixtures" Metals 6, no. 8: 190. https://doi.org/10.3390/met6080190