Barrier Graphene Oxide on a CoCr Alloy via Silane/GO Covalent Bonding and Its Electrochemical Behavior in a Simulated Synovial Fluid Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
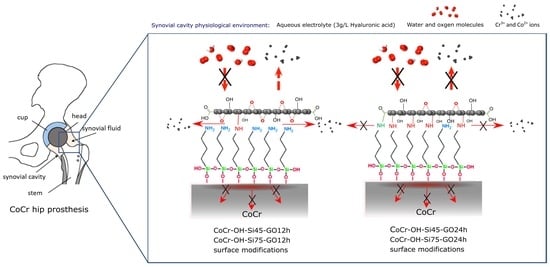
2.2. Preparation of Multilayer Systems
2.3. Electrochemical Characterization: Electrochemical Impedance Spectroscopy (EIS)
3. Results
3.1. Bare CoCr and CoCr-OH
3.2. Silanized CoCr-OH Samples (CoCr-OH-Si)
3.3. Graphene Oxide on Silanized CoCr-OH Samples (CoCr-OH-Si-GO)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Milošev, I. CoCrMo Alloy for Biomedical Applications. In Biomedical Applications. Modern Aspects of Electrochemistry; Djokić, S., Ed.; Springer: Boston, MA, USA, 2012; Volume 55, pp. 1–72. [Google Scholar] [CrossRef]
- Taghizadeh, B.; Ghavami, L.; Derakhshankhah, H.; Zangene, E.; Razmi, M.; Jaymand, M.; Zarrintaj, P.; Zarghami, N.; Jaafari, M.R.; Shahri, M.M.; et al. Biomaterials in Valvular Heart Diseases. Front. Bioeng. Biotechnol. 2020, 8, 529244. [Google Scholar] [CrossRef]
- Milošev, I.; Strehblow, H.-H. The composition of the surface passive film formed on CoCrMo alloy in simulated physiological solution. Electrochim. Acta 2003, 48, 2767–2774. [Google Scholar] [CrossRef]
- Arnholt, C.M.; MacDonald, D.W.; Malkani, A.L.; Klein, G.R.; Rimnac, C.M.; Kurtz, S.M.; Kocagoz, S.B.; Gilbert, J.L. Corrosion Damage and Wear Mechanisms in Long-Term Retrieved CoCr Femoral Components for Total Knee Arthroplasty. J. Arthroplast. 2016, 31, 2900–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichenbaum, G.; Wilsey, J.T.; Fessel, G.; Qiu, Q.-Q.; Perkins, L.; Hasgall, P.; Monnot, A.; More, S.L.; Egnot, N.; Sague, J.; et al. An integrated benefit-risk assessment of cobalt-containing alloys used in medical devices: Implications for regulatory requirements in the European Union. Regul. Toxicol. Pharmacol. 2021, 125, 105004. [Google Scholar] [CrossRef]
- Halwani, D.O.; Anderson, P.G.; Lemons, J.E.; Jordan, W.D.; Anayiotos, A.S.; Brott, B.C. In-vivo corrosion and local release of metallic ions from vascular stents into surrounding tissue. J. Invasive Cardiol. 2010, 22, 528. [Google Scholar] [PubMed]
- Matusiewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles—A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef] [PubMed]
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.; Macaluso, F.; Cobelli, N.; et al. Molecular analysis of chromium and cobalt-related toxicity. Sci. Rep. 2015, 4, 5729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posada, O.M.; Gilmour, D.; Tate, R.J.; Grant, M.H. CoCr wear particles generated from CoCr alloy metal-on-metal hip replacements, and cobalt ions stimulate apoptosis and expression of general toxicology-related genes in monocyte-like U937 cells. Toxicol. Appl. Pharmacol. 2014, 281, 125–135. [Google Scholar] [CrossRef]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef]
- Bijukumar, D.R.; Segu, A.; de Souza, J.C.M.; Li, X.; Barba, M.; Mercuri, L.G.; Jacobs, J.J.; Mathew, M.T. Systemic and local toxicity of metal debris released from hip prostheses: A review of experimental approaches. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 951–963. [Google Scholar] [CrossRef]
- Eltit, F.; Noble, J.; Sharma, M.; Benam, N.; Haegert, A.; Bell, R.H.; Simon, F.; Duncan, C.P.; Garbuz, D.S.; Greidanus, N.V.; et al. Cobalt ions induce metabolic stress in synovial fibroblasts and secretion of cytokines/chemokines that may be diagnostic markers for adverse local tissue reactions to hip implants. Acta Biomater. 2021, 131, 581–594. [Google Scholar] [CrossRef]
- Kwon, Y.-M.; Tsai, T.-Y.; Leone, W.A.; Liow, M.H.L. Sensitivity and Specificity of Metal Ion Levels in Predicting “Pseudotumors” due to Taper Corrosion in Patients with Dual Taper Modular Total Hip Arthroplasty. J. Arthroplast. 2017, 32, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Matharu, G.S.; Berryman, F.; Judge, A.; Reito, A.; McConnell, J.; Lainiala, O.; Young, S.; Eskelinen, A.; Pandit, H.G.; Murray, D.W. Blood Metal Ion Thresholds to Identify Patients with Metal-on-Metal Hip Implants at Risk of Adverse Reactions to Metal Debris: An External Multicenter Validation Study of Birmingham Hip Resurfacing and Corail-Pinnacle Implants. J. Bone Jt. Surg. 2017, 99, 1532–1539. [Google Scholar] [CrossRef] [Green Version]
- van der Weegen, W.; Sijbesma, T.; Hoekstra, H.J.; Brakel, K.; Pilot, P.; Nelissen, R.G. Treatment of Pseudotumors after Metal-on-Metal Hip Resurfacing Based on Magnetic Resonance Imaging, Metal Ion Levels and Symptoms. J. Arthroplast. 2014, 29, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Sabah, S.A.; Bandi, A.S.; Maggiore, P.; Tarassoli, P.; Sampson, B.; Skinner, J.A.; Markel, D.C.; Bou-Akl, T.; Rossi, M.D.; Pizzimenti, N.; et al. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J. Bone Jt. Surg. Br 2011, 93-B, 1308–1313. [Google Scholar] [CrossRef] [Green Version]
- De Smet, K.; De Haan, R.; Calistri, A.; Campbell, P.; Ebramzadeh, E.; Pattyn, C.; Gill, H. Metal Ion Measurement as a Diagnostic Tool to Identify Problems with Metal-on-Metal Hip Resurfacing. J. Bone Jt. Surg. 2008, 90, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Berry, V. Impermeability of graphene and its applications. Carbon 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; Van Der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable Atomic Membranes from Graphene Sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [Green Version]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Graphene: A perfect nanoballoon. Appl. Phys. Lett. 2008, 93, 193107. [Google Scholar] [CrossRef] [Green Version]
- Sagade, A.A.; Aria, A.I.; Edge, S.; Melgari, P.; Gieseking, B.; Bayer, B.C.; Meyer, J.C.; Bird, D.; Brewer, P.; Hofmann, S. Graphene-based nanolaminates as ultra-high permeation barriers. npj 2D Mater. Appl. 2017, 1, 35. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.-K.; Park, M.-H.; Kim, Y.-H.; Kwon, S.-J.; Jeong, S.-H.; Lee, T.-W. Laminated Graphene Films for Flexible Transparent Thin Film Encapsulation. ACS Appl. Mater. Interfaces 2016, 8, 14725–14731. [Google Scholar] [CrossRef] [PubMed]
- Topsakal, M.; Sahin, H.; Ciraci, S. Graphene coatings: An efficient protection from oxidation. Phys. Rev. B 2012, 85, 155445. [Google Scholar] [CrossRef] [Green Version]
- Ding, R.; Li, W.; Wang, X.; Gui, T.; Li, B.; Han, P.; Tian, H.; Liu, A.; Wang, X.; Liu, X.; et al. A brief review of corrosion protective films and coatings based on graphene and graphene oxide. J. Alloys Compd. 2018, 764, 1039–1055. [Google Scholar] [CrossRef]
- Malhotra, R.; Han, Y.; Nijhuis, C.A.; Silikas, N.; Neto, A.C.; Rosa, V. Graphene nanocoating provides superb long-lasting corrosion protection to titanium alloy. Dent. Mater. 2021, 37, 1553–1560. [Google Scholar] [CrossRef]
- Amin, I.; Batyrev, E.; de Vooys, A.; van der Weijde, H.; Shiju, N.R. Covalent polymer functionalization of graphene/graphene oxide and its application as anticorrosion materials. 2D Mater. 2022, 9, 032002. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Fan, T.; Zhang, D. Metal-graphene interfaces in epitaxial and bulk systems: A review. Prog. Mater. Sci. 2020, 110, 100652. [Google Scholar] [CrossRef]
- Kozlov, S.M.; Viñes, F.; Görling, A. Bonding Mechanisms of Graphene on Metal Surfaces. J. Phys. Chem. C 2012, 116, 7360–7366. [Google Scholar] [CrossRef]
- Weatherup, R.S.; D’arsié, L.; Cabrero-Vilatela, A.; Caneva, S.; Blume, R.; Robertson, J.; Schloegl, R.; Hofmann, S. Long-Term Passivation of Strongly Interacting Metals with Single-Layer Graphene. J. Am. Chem. Soc. 2015, 137, 14358–14366. [Google Scholar] [CrossRef] [Green Version]
- Morrow, W.K.; Pearton, S.J.; Ren, F. Review of Graphene as a Solid State Diffusion Barrier. Small 2016, 12, 120–134. [Google Scholar] [CrossRef]
- Yin, C.; Du, X.; Ding, Z.; Zeng, Q.; Li, X.; He, C.; Xiong, B.; Li, J.; Zhou, Y. Gas permeation and microstructure of reduced graphene oxide/polyethyleneimine multilayer films created via recast and layer-by-layer deposition processes. RSC Adv. 2022, 12, 6561–6572. [Google Scholar] [CrossRef]
- Khosravi, I.; Shahryari, Z.; Moghadas, S.M.J.; Sarraf, H.T.; Yeganeh, M. Chapter 8—The application of graphene oxide as corrosion barrier. In Corrosion Protection at the Nanoscale; Rajendran, S., Nguyen, T.A., Kakooei, S., Yeganeh, M., Li, Y., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 127–140. [Google Scholar] [CrossRef]
- Chae, W.H.; Sannicolo, T.; Grossman, J.C. Double-Sided Graphene Oxide Encapsulated Silver Nanowire Transparent Electrode with Improved Chemical and Electrical Stability. ACS Appl. Mater. Interfaces 2020, 12, 17909–17920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruben, B.; Zhang, G.; Xin, T.; Giorgio, S.; Victor, M.; Gloria, G.; Michele, F.; Filippo, P.; Shuhui, S.; Nadhira, L.; et al. Graphene oxide/reduced graphene oxide films as protective barriers on lead against differential aeration corrosion induced by water drops. Nanoscale Adv. 2020, 2, 5412–5420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xie, X.; Zhang, C. Effect of the graphene oxide additive on the corrosion resistance of the plasma electrolytic oxidation coating of the AZ31 magnesium alloy. Corros. Sci. 2017, 114, 146–155. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ghasemi, E.; Mahdavian, M.; Changizi, E.; Moghadam, M.M. Covalently-grafted graphene oxide nanosheets to improve barrier and corrosion protection properties of polyurethane coatings. Carbon 2015, 93, 555–573. [Google Scholar] [CrossRef]
- Hu, Y.; Li, S.; Kang, W.; Lin, H.; Hu, Y. Surface modification of Ti6Al4V alloy by polydopamine grafted GO/ZnO nanocomposite coating. Surf. Coat. Technol. 2021, 422, 127534. [Google Scholar] [CrossRef]
- Lee, W.-J.; Kim, C.-S.; Yang, S.-Y.; Lee, D.; Kim, Y.-S. Ultrathin Super-barrier film via 100 % surface coating coverage of Self-assembled graphene oxide sheets. Chem. Eng. J. 2022, 440, 135913. [Google Scholar] [CrossRef]
- Srimaneepong, V.; Rokaya, D.; Thunyakitpisal, P.; Qin, J.; Saengkiettiyut, K. Corrosion Resistance of Graphene oxide/Silver Coatings on Ni–Ti alloy and Expression of IL-6 and IL-8 in Human Oral Fibroblasts. Sci. Rep. 2020, 10, 3247. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Kravets, V.G.; Wong, S.L.; Waters, J.; Geim, A.K.; Nair, R.R. Impermeable barrier films and protective coatings based on reduced graphene oxide. Nat. Commun. 2014, 5, 4843. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, H.; Granstrom, J.; Nie, W.; Sojoudi, H.; Fujita, T.; Voiry, D.; Chen, M.; Gupta, G.; Mohite, A.D.; Graham, S.; et al. Reduced Graphene Oxide Thin Films as Ultrabarriers for Organic Electronics. Adv. Energy Mater. 2014, 4, 1300986. [Google Scholar] [CrossRef] [Green Version]
- Rahpeima, S.; Dief, E.M.; Peiris, C.R.; Ferrie, S.; Duan, A.; Ciampi, S.; Raston, C.L.; Darwish, N. Reduced graphene oxide–silicon interface involving direct Si–O bonding as a conductive and mechanical stable ohmic contact. Chem. Commun. 2020, 56, 6209–6212. [Google Scholar] [CrossRef]
- Rahpeima, S.; Dief, E.M.; Ciampi, S.; Raston, C.L.; Darwish, N. Impermeable Graphene Oxide Protects Silicon from Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 38799–38807. [Google Scholar] [CrossRef]
- Sánchez-López, L.; Chico, B.; Llorente, I.; Escudero, M.L.; Lozano, R.M.; García-Alonso, M.C. Covalent immobilization of graphene oxide on biomedical grade CoCr alloy by an improved multilayer system assembly via Silane/GO bonding. Mater. Chem. Phys. 2022, 28, 126296. [Google Scholar] [CrossRef]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.M.; Zuilhof, H. Covalent Surface Modification of Oxide Surfaces. Angew. Chem. Int. Ed. 2014, 53, 6322–6356. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, F.; Shan, C.; Han, D.; Zhang, Q.; Niu, L.; Ivaska, A. Covalent functionalization of chemically converted graphene sheets via silane and its reinforcement. J. Mater. Chem. 2009, 19, 4632–4638. [Google Scholar] [CrossRef]
- Serodre, T.; Oliveira, N.; Miquita, D.R.; Ferreira, M.; Santos, A.; Resende, V.; Furtado, C. Surface Silanization of Graphene Oxide Under Mild Reaction Conditions. J. Braz. Chem. Soc. 2019, 30, 2488–2499. [Google Scholar] [CrossRef]
- Mazzucco, D.; Scott, R.; Spector, M. Composition of joint fluid in patients undergoing total knee replacement and revision arthroplasty: Correlation with flow properties. Biomaterials 2004, 25, 4433–4445. [Google Scholar] [CrossRef]
- Barranco, V.; Escudero, M.L.; García, M.C. 3D, Chemical and Electrochemical characterization of blasted Ti6Al4V surfaces: Its influence on the corrosion behaviour. Electrochim. Acta 2007, 52, 4374–4384. [Google Scholar] [CrossRef] [Green Version]
- García-Alonso, M.C.; Saldaña, L.; Alonso, C.; Barranco, V.; Muñoz-Morris, M.A.; Escudero, M.L. In situ cell culture monitoring on Ti6Al4V surface by electrochemical techniques. Acta Biomater. 2009, 5, 1374–1383. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Escudero, M.; Llorente, I.; Pérez-Maceda, B.; José-Pinilla, S.S.; Sánchez-López, L.; Lozano, R.; Aguado-Henche, S.; de Arriba, C.C.; Alobera-Gracia, M.; García-Alonso, M. Electrochemically reduced graphene oxide on CoCr biomedical alloy: Characterization, macrophage biocompatibility and hemocompatibility in rats with graphene and graphene oxide. Mater. Sci. Eng. C 2020, 109, 110522. [Google Scholar] [CrossRef]
- García-Alonso, M.C.; Llorente, I.; Díaz, I.; Escudero, M.L. Interaction of Hyaluronic Acid with CoCr Alloy Under Immersion and Wear–Corrosion Processes. Tribol. Lett. 2018, 66, 122. [Google Scholar] [CrossRef]
- Hanawa, T.; Hiromoto, S.; Asami, K. Characterization of the surface oxide film of a Co–Cr–Mo alloy after being located in quasi-biological environments using XPS. Appl. Surf. Sci. 2001, 183, 68. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Okazaki, Y.; Gotoh, E. Metal release from stainless steel, Co–Cr–Mo–Ni–Fe and Ni–Ti alloys in vascular implants. Corros. Sci. 2008, 50, 3429–3438. [Google Scholar] [CrossRef]
- Davda, K.; Lali, F.V.; Sampson, B.; Skinner, J.A.; Hart, A.J. An analysis of metal ion levels in the joint fluid of symptomatic patients with metal-on-metal hip replacements. J. Bone Jt. Surg. Br 2011, 93-B, 738–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosman, A.H.; van der Mei, H.C.; Bulstra, S.K.; Busscher, H.J.; Neut, D. Effects of metal-on-metal wear on the host immune system and infection in hip arthroplasty. Acta Orthop. 2010, 81, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Contu, F.; Elsener, B.; Böhni, H. Electrochemical Behavior of CoCrMo Alloy in the Active State in Acidic and Alkaline Buffered Solutions. J. Electrochem. Soc. 2003, 150, B419. [Google Scholar] [CrossRef]
- Franquet, A.; Le Pen, C.; Terryn, H.; Vereecken, J. Effect of bath concentration and curing time on the structure of non-functional thin organosilane layers on aluminium. Electrochim. Acta 2003, 48, 1245–1255. [Google Scholar] [CrossRef]
- Marcinko, S.; Fadeev, A.Y. Hydrolytic Stability of Organic Monolayers Supported on TiO2 and ZrO2. Langmuir 2004, 20, 2270–2273. [Google Scholar] [CrossRef]
- Xiao, S.J.; Textor, M.; Spencer, N.D.; Sigrist, H. Covalent Attachment of Cell-Adhesive, (Arg-Gly-Asp)-Containing Peptides to Titanium Surfaces. Langmuir 1998, 14, 5507–5516. [Google Scholar] [CrossRef]
- Rajaura, R.S.; Srivastava, S.; Sharma, V.; Sharma, P.; Lal, C.; Singh, M.; Palsania, H.; Vijay, Y. Role of interlayer spacing and functional group on the hydrogen storage properties of graphene oxide and reduced graphene oxide. Int. J. Hydrogen Energy 2016, 41, 9454–9461. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded Permeation of Water through Helium-Leak–Tight Graphene-Based Membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [Green Version]
- Mi, B. Graphene Oxide Membranes for Ionic and Molecular Sieving. Science 2014, 343, 740–742. [Google Scholar] [CrossRef]
- Hegab, H.M.; Kallem, P.; Pandey, R.P.; Ouda, M.; Banat, F.; Hasan, S.W. Mechanistic insights into the selective mass-transport and fabrication of holey graphene-based membranes for water purification applications. Chem.Eng. J. 2022, 431, 134248. [Google Scholar] [CrossRef]
- Qi, B.; He, X.; Zeng, G.; Pan, Y.; Li, G.; Liu, G.; Zhang, Y.; Chen, W.; Sun, Y. Strict molecular sieving over electrodeposited 2D-interspacing-narrowed graphene oxide membranes. Nat. Commun. 2017, 8, 825. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Zhu, M.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Xu, Z.; Zhu, H. Selective Ion Penetration of Graphene Oxide Membranes. ACS Nano 2013, 7, 428–437. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [Green Version]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef]
- Xi, Y.-H.; Liu, Z.; Ji, J.; Wang, Y.; Faraj, Y.; Zhu, Y.; Xie, R.; Ju, X.-J.; Wang, W.; Lu, X.; et al. Graphene-based membranes with uniform 2D nanochannels for precise sieving of mono-/multi-valent metal ions. J. Membr. Sci. 2018, 550, 208–218. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.; Lee, H.G.; Jin, D.; Kim, G.; Kim, T.; Lee, J.S.; Shim, W. Neuromorphic van der Waals crystals for substantial energy generation. Nat. Commun. 2021, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Tansel, B.; Sager, J.; Rector, T.; Garland, J.; Strayer, R.F.; Levine, L.; Roberts, M.; Hummerick, M.; Bauer, J. Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep. Purif. Technol. 2006, 51, 40–47. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, K.; Ji, Y.; Liu, G.; Jin, W.; Xu, N. Controllable ion transport by surface-charged graphene oxide membrane. Nat. Commun. 2019, 10, 1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Kielland, J. Individual Activity Coefficients of Ions in Aqueous Solutions. J. Am. Chem. Soc. 1937, 59, 1675–1678. [Google Scholar] [CrossRef]
- Zhang, W.; Lee, S.; McNear, K.L.; Chung, T.-F.; Lee, S.; Lee, K.; Crist, S.A.; Ratliff, T.L.; Zhong, Z.; Chen, Y.P.; et al. Use of graphene as protection film in biological environments. Sci. Rep. 2014, 4, 4097. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Liu, X.; Xu, Z.; Ren, H.; Deng, B.; Tang, M.; Lu, L.; Fu, X.; Peng, H.; Liu, Z.; et al. Graphene Encapsulated Copper Microwires as Highly MRI Compatible Neural Electrodes. Nano Lett. 2016, 16, 7731–7738. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, R.; Han, Y.; Morin, J.; Luong-Van, E.; Chew, R.; Neto, A.C.; Nijhuis, C.; Rosa, V. Inhibiting Corrosion of Biomedical-Grade Ti-6Al-4V Alloys with Graphene Nanocoating. J. Dent. Res. 2021, 99, 285–292. [Google Scholar] [CrossRef]




| Time, d | RS ± abs. Error, Ω | RL ± abs. Error, MΩ | n ± abs. Error | Ceff, µF | Chi2 | ||
|---|---|---|---|---|---|---|---|
| CoCr | 0 | 1795 ± 21.54 | 45 ± 0.63 | 11.2 ± 0.01 | 0.93 ± 0.0004 | 2.24 | 1.34 × |
| 1 | 1558 ± 24.30 | 98 ± 2.82 | 10.3 ± 0.01 | 0.94 ± 0.0004 | 2.74 | 1.55 × | |
| 2 | 1359 ± 7.75 | 138 ± 3.48 | 10.6 ± 0.02 | 0.95 ± 0.0006 | 3.69 | 4.13 × | |
| 3 | 1174 ± 1.76 | 241 ± 10.72 | 10.6 ± 0.02 | 0.95 ± 0.0005 | 3.74 | 2.17 × | |
| 4 | 1447 ± 2.89 | 224 ± 8.18 | 10.2 ± 0.01 | 0.95 ± 0.0004 | 3.58 | 3.44 × | |
| 7 | 1307 ± 1.96 | 282 ± 9.14 | 10.2 ± 0.01 | 0.96 ± 0.0003 | 4.49 | 2.01 × | |
| CoCr-OH | 0 | 3574 ± 9.29 | 1.87 ± 0.13 | 14.9 ± 0.29 | 0.91 ± 0.0012 | 1.35 | 4.43 × |
| 1 | 3495 ± 13.98 | 8.45 ± 0.14 | 12.2 ± 0.39 | 0.92 ± 0.0014 | 1.65 | 2.44 × | |
| 2 | 3514 ± 19.68 | 10.5 ± 0.26 | 11.1 ± 0.53 | 0.92 ± 0.0019 | 1.52 | 4.73 × | |
| 3 | 3224 ± 18.38 | 12.4 ± 0.33 | 10.7 ± 0.53 | 0.93 ± 0.0019 | 1.94 | 4.85 × | |
| 4 | 3075 ± 15.99 | 15.5 ± 0.42 | 10.3 ± 0.48 | 0.93 ± 0.0017 | 1.86 | 4.05 × | |
| 7 | 3332 ± 16.33 | 28.8 ± 1.14 | 9.77 ± 0.46 | 0.94 ± 0.0015 | 2.40 | 3.69 × |
| Time, d | RS ± abs. Error, Ω | RCOAT ± abs. Error, Ω | n2 ± abs. Error | RL ± abs. Error, MΩ | n1 ± abs. Error | Chi2 | |||
|---|---|---|---|---|---|---|---|---|---|
| CoCr-OH-Si45 | 0 | 3385 ± 71.67 | 13.8 × 106 ± 0.14 × 106 | 8.3 ± 0.15 | 0.88 ± 0.007 | 6.24 × | |||
| 1 | 3326 ± 18.14 | 746.6 ± 87.02 | 21.63 ± 8.28 | 0.89 ± 0.08 | 15.5 ± 0.04 | 12.9 ± 0.06 | 0.94 ± 0.002 | 2.33 × | |
| 2 | 3079 ± 23.33 | 718.3 ± 128.9 | 20.34 ± 10.1 | 0.97 ± 0.11 | 17.5 ± 0.07 | 12.3 ± 0.08 | 0.94 ± 0.003 | 5.43 × | |
| 3 | 2117 ± 9.89 | 632.3 ± 56.1 | 30.32 ± 8.26 | 0.81 ± 0.05 | 16.8 ± 0.03 | 12.6 ± 0.04 | 0.94 ± 0.001 | 1.34 × | |
| 4 | 2456 ± 7.49 | 811.8 ± 48.1 | 33.01 ± 5.43 | 0.79 ± 0.03 | 24.7 ± 0.04 | 12.5 ± 0.03 | 0.95 ± 0.001 | 5.83 × | |
| 7 | 2827 ± 5.53 | 1008 ± 38.8 | 34.07 ± 3.36 | 0.78 ± 0.02 | 37.0 ± 1.34 | 12.3 ± 0.06 | 0.95 ± 0.001 | 2.46 × | |
| CoCr-OH-Si75 | 0 | 3083 ± 13.60 | 10.6 × 106 ± 0.26 × 106 | 14.9 ± 0.06 | 0.92 ± 0.002 | 2.98 × | |||
| 1 | 2081 ± 19.23 | 6.63 × 106 ± 24.5 × 106 | 15.26 ± 0.13 | 0.91 ± 0.003 | 1.23 × | ||||
| 2 | 2257 ± 21.55 | 233.2 ± 74.52 | 26.98 ± 36.18 | 0.94 ± 0.25 | 7.16 ± 0.19 | 14.5 ± 0.10 | 0.92 ± 0.003 | 6.66 × | |
| 3 | 2299 ± 23.47 | 238.2 ± 80.96 | 25.66 ± 36.62 | 0.95 ± 0.26 | 6.33 ± 0.17 | 14.2 ± 0.10 | 0.92 ± 0.003 | 7.71 × | |
| 4 | 2312 ± 19.85 | 246.6 ± 69.58 | 30.22 ± 35.55 | 0.91 ± 0.21 | 7.81 ± 0.18 | 13.9 ± 0.08 | 0.93 ± 0.002 | 5.02 × | |
| 7 | 2420 ± 17.87 | 355 ± 79.35 | 36.76 ± 28.93 | 0.87 ± 0.15 | 9.27 ± 0.21 | 13.7 ± 0.07 | 0.93 ± 0.002 | 3.73 × |
| Time, d | Rs ± abs. Error, Ω | RCOAT ± abs. Error, Ω | n2 ± abs. Error | RL ± abs. Error, MΩ | n1 ± abs. Error | Chi2 | |||
|---|---|---|---|---|---|---|---|---|---|
| CoCr-OH-Si45-GO12h | 0 | 3638 ± 10.89 | 1124 ± 125.9 | 40.4 ± 8.12 | 0.84 ± 0.04 | 54.1 ± 0.20 | 9.4 ± 0.03 | 0.92 ± 0.001 | 7.48 × |
| 1 | 3845 ± 5.32 | 2396 ± 81.56 | 30.1 ± 1.55 | 0.81 ± 0.01 | 53.2 ± 0.96 | 10.5 ± 0.01 | 0.93 ± 0.001 | 1.64 × | |
| 2 | 3776 ± 5.44 | 2427 ± 75.26 | 28.6 ± 1.40 | 0.81 ± 0.01 | 45.4 ± 0.75 | 11.2 ± 0.02 | 0.93 ± 0.003 | 1.77 × | |
| 3 | 3458 ± 5.12 | 2381 ± 73.04 | 29.2 ± 1.41 | 0.80 ± 0.01 | 47.2 ± 0.80 | 11.2 ± 0.06 | 0.94 ± 0.002 | 1.80 × | |
| 4 | 3190 ± 4.82 | 2250 ± 70.36 | 31.3 ± 1.54 | 0.79 ± 0.01 | 48.1 ± 0.81 | 11.2 ± 0.02 | 0.94 ± 0.001 | 1.78 × | |
| 7 | 3139 ± 4.06 | 2302 ± 60.35 | 31.5 ± 1.29 | 0.78 ± 0.01 | 57.9 ± 0.96 | 12.3 ± 0.04 | 0.95 ± 0.001 | 1.28 × | |
| CoCr-OH-Si45-GO24h | 0 | 3328 ± 7.78 | 3532 ± 281.44 | 73.3 ± 4.49 | 0.68 ± 0.02 | 66.5 ± 0.35 | 16.0 ± 0.05 | 0.94 ± 0.001 | 4.02 × |
| 1 | 3808 ± 8.48 | 5017 ± 360.36 | 61.5 ± 2.98 | 0.68 ± 0.01 | 73.0 ± 0.43 | 15.8 ± 0.06 | 0.93 ± 0.001 | 3.83 × | |
| 2 | 3860 ± 9.49 | 4822 ± 369.67 | 58.9 ± 3.25 | 0.69 ± 0.01 | 73.4 ± 0.46 | 15.37 ± 0.06 | 0.93 ± 0.001 | 4.69 × | |
| 3 | 3825 ± 8.94 | 4822 ± 344.78 | 59.0 ± 3.08 | 0.68 ± 0.01 | 81.9 ± 0.52 | 15.30 ± 0.06 | 0.94 ± 0.001 | 4.13 × | |
| 4 | 3751 ± 8.79 | 4756 ± 341.84 | 59.7 ± 3.13 | 0.68 ± 0.01 | 84.4 ± 0.54 | 15.15 ± 0.05 | 0.94 ± 0.001 | 4.11 × | |
| 7 | 3449 ± 8.63 | 4549 ± 346.76 | 61.6 ± 3.42 | 0.67 ± 0.01 | 81.9 ± 0.52 | 14.96 ± 0.05 | 0.94 ± 0.001 | 4.49 × | |
| CoCr-OH-Si75-GO12h | 0 | 1668 ± 7.57 | 1127 ± 370.87 | 229.3 ± 51.3 | 0.59 ± 0.05 | 44.8 ± 0.24 | 14.3 ± 0.06 | 0.91 ± 0.002 | 8.65 × |
| 1 | 2647 ± 8.73 | 262.5 ± 63.68 | 128.9 ± 76.4 | 0.77 ± 0.12 | 42.3 ± 0.14 | 12.0 ± 0.03 | 0.93 ± 0.001 | 6.74 × | |
| 2 | 1687 ± 6.97 | 255 ± 74.24 | 159.8 ± 92.6 | 0.75 ± 0.11 | 37.0 ± 0.12 | 11.5 ± 0.03 | 0.93 ± 0.001 | 9.74 × | |
| 3 | 1592 ± 5.95 | 270.9 ± 68.66 | 167.2 ± 80.4 | 0.73 ± 0.09 | 44.7 ± 0.14 | 11.2 ± 0.03 | 0.94 ± 0.001 | 7.47 × | |
| 4 | 1502 ± 5.29 | 268.5 ± 61.80 | 170.5 ± 74.2 | 0.72 ± 0.08 | 44.9 ± 0.13 | 11.1 ± 0.03 | 0.94 ± 0.001 | 6.31 × | |
| 7 | 1397 ± 6.31 | 276.7 ± 77.17 | 176.2 ± 90.5 | 0.70 ± 0.09 | 36.9 ± 0.10 | 10.8 ± 0.03 | 0.94 ± 0.001 | 9.47 × | |
| CoCr-OH-Si75-GO24h | 0 | 3250 ± 5.42 | 377.1 ± 81.07 | 289.2 ± 88.3 | 0.66 ± 0.06 | 109.8 ± 0.38 | 11.3 ± 0.02 | 0.94 ± 0.002 | 1.51 × |
| 1 | 3504 ± 11.15 | 122.3 ± 53.94 | 194.9 ± 271.5 | 0.77 ± 0.26 | 64.0 ± 0.24 | 10.7 ± 0.02 | 0.93 ± 0.002 | 5.64 × | |
| 2 | 3573 ± 12.79 | 91.58 ± 51.24 | 191.9 ± 387.7 | 0.78 ± 0.37 | 59.0 ± 0.22 | 10.3 ± 0.02 | 0.94 ± 0.003 | 6.89 × | |
| 3 | 3690 ± 12.17 | 92.76 ± 50.83 | 180.2 ± 348.5 | 0.81 ± 0.36 | 60.7 ± 0.22 | 10.2 ± 0.02 | 0.94 ± 0.002 | 6.69 × | |
| 4 | 3683 ± 12.55 | 92.5 ± 50.85 | 170.1 ± 337.7 | 0.82 ± 0.37 | 58.5 ± 0.21 | 10.1 ± 0.02 | 0.94 ± 0.001 | 7.12 × | |
| 7 | 3459 ± 12.08 | 73.1 ± 43.26 | 157.5 ± 370.6 | 0.84 ± 0.43 | 50.8 ± 0.16 | 10.0 ± 0.02 | 0.94 ± 0.001 | 7.42 × |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-López, L.; Chico, B.; Escudero, M.L.; Lozano, R.M.; García-Alonso, M.C. Barrier Graphene Oxide on a CoCr Alloy via Silane/GO Covalent Bonding and Its Electrochemical Behavior in a Simulated Synovial Fluid Electrolyte. Metals 2023, 13, 1331. https://doi.org/10.3390/met13081331
Sánchez-López L, Chico B, Escudero ML, Lozano RM, García-Alonso MC. Barrier Graphene Oxide on a CoCr Alloy via Silane/GO Covalent Bonding and Its Electrochemical Behavior in a Simulated Synovial Fluid Electrolyte. Metals. 2023; 13(8):1331. https://doi.org/10.3390/met13081331
Chicago/Turabian StyleSánchez-López, Luna, Belén Chico, María Lorenza Escudero, Rose María Lozano, and María Cristina García-Alonso. 2023. "Barrier Graphene Oxide on a CoCr Alloy via Silane/GO Covalent Bonding and Its Electrochemical Behavior in a Simulated Synovial Fluid Electrolyte" Metals 13, no. 8: 1331. https://doi.org/10.3390/met13081331